IL-1R1 signaling regulates CXCL12-mediated T cell localization and fate within the central nervous system during West Nile Virus encephalitis
- PMID: 25200953
- PMCID: PMC4340598
- DOI: 10.4049/jimmunol.1401192
IL-1R1 signaling regulates CXCL12-mediated T cell localization and fate within the central nervous system during West Nile Virus encephalitis
Abstract
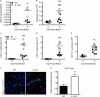
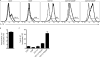
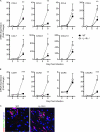
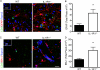
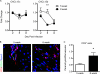
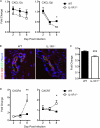
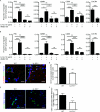
Immune cell entry into the virally infected CNS is vital for promoting viral clearance yet may contribute to neuropathology if not rigorously regulated. We previously showed that signaling through IL-1R1 is critical for effector T cell reactivation and virologic control within the CNS during murine West Nile virus (WNV) encephalitis. WNV-infected IL-1R1(-/-) mice also display increased parenchymal penetration of CD8(+) T cells despite lack of CD4-mediated full activation, suggesting dysregulation of molecular components of CNS immune privilege. In this study, we show that IL-1 signaling regulates the CNS entry of virus-specific lymphocytes, promoting protective immune responses to CNS viral infections that limit immunopathology. Analysis of blood-brain barrier function in the WNV-infected IL-1R1(-/-) mice revealed no alterations in permeability. However, parenchymal proinflammatory chemokine expression, including CCL2, CCL5, and CXCL10, was significantly upregulated, whereas microvasculature CXCL12 expression was significantly decreased in the absence of IL-1 signaling. We show that during WNV infection, CD11b(+)CD45(hi) infiltrating cells (macrophages) are the primary producers of IL-1β within the CNS and, through the use of an in vitro blood-brain barrier model, that IL-1β promotes CXCR4-mediated T cell adhesion to brain microvasculature endothelial cells. Of interest, IFNγ(+) and CD69(+) WNV-primed T cells were able to overcome CXCL12-mediated adhesion via downregulation of CXCR4. These data indicate that infiltrating IL-1β-producing leukocytes contribute to cellular interactions at endothelial barriers that impart protective CNS inflammation by regulating the parenchymal entry of CXCR4(+) virus-specific T cells during WNV infection.
Copyright © 2014 by The American Association of Immunologists, Inc.
Figures

References
-
- McCandless EE, Wang Q, Woerner BM, Harper JM, Klein RS. CXCL12 limits inflammation by localizing mononuclear infiltrates to the perivascular space during experimental autoimmune encephalomyelitis. J Immunol. 2006;177:8053–8064. - PubMed
-
- Bouffard JP, Riudavets MA, Holman R, Rushing EJ. Neuropathology of the brain and spinal cord in human West Nile virus infection. Clinical neuropathology. 2004;23:59–61. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

