IRAK4 dimerization and trans-autophosphorylation are induced by Myddosome assembly
- PMID: 25201411
- PMCID: PMC4169746
- DOI: 10.1016/j.molcel.2014.08.006
IRAK4 dimerization and trans-autophosphorylation are induced by Myddosome assembly
Abstract
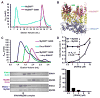
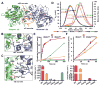
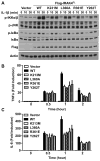
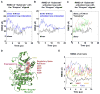
Trans-autophosphorylation is among the most prevalent means of protein kinase activation, yet its molecular basis is poorly defined. In Toll-like receptor and interleukin-1 receptor signaling pathways, the kinase IRAK4 is recruited to the membrane-proximal adaptor MyD88 through death domain (DD) interactions, forming the oligomeric Myddosome and mediating NF-κB activation. Here we show that unphosphorylated IRAK4 dimerizes in solution with a KD of 2.5 μM and that Myddosome assembly greatly enhances IRAK4 kinase domain (KD) autophosphorylation at sub-KD concentrations. The crystal structure of the unphosphorylated IRAK4(KD) dimer captures a conformation that appears to represent the actual trans-autophosphorylation reaction, with the activation loop phosphosite of one IRAK4 monomer precisely positioned for phosphotransfer by its partner. We show that dimerization is crucial for IRAK4 autophosphorylation in vitro and ligand-dependent signaling in cells. These studies identify a mechanism for oligomerization-driven allosteric autoactivation of IRAK4 that may be general to other kinases activated by autophosphorylation.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures



Comment in
-
IRAK4 activation: a cautious embrace.Mol Cell. 2014 Sep 18;55(6):805-806. doi: 10.1016/j.molcel.2014.09.003. Mol Cell. 2014. PMID: 25238194
References
-
- Cai Z, Chehab NH, Pavletich NP. Structure and activation mechanism of the CHK2 DNA damage checkpoint kinase. Mol Cell. 2009;35:818–829. - PubMed
-
- Cheng H, Addona T, Keshishian H, Dahlstrand E, Lu C, Dorsch M, Li Z, Wang A, Ocain TD, Li P, et al. Regulation of IRAK-4 kinase activity via autophosphorylation within its activation loop. Biochem Biophys Res Commun. 2007;352:609–616. - PubMed
-
- Favelyukis S, Till JH, Hubbard SR, Miller WT. Structure and autoregulation of the insulin-like growth factor 1 receptor kinase. Nat Struct Biol. 2001;8:1058–1063. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

