Mapping the zoonotic niche of Ebola virus disease in Africa
- PMID: 25201877
- PMCID: PMC4166725
- DOI: 10.7554/eLife.04395
Mapping the zoonotic niche of Ebola virus disease in Africa
Abstract
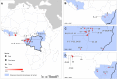
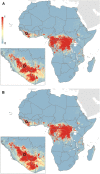
Ebola virus disease (EVD) is a complex zoonosis that is highly virulent in humans. The largest recorded outbreak of EVD is ongoing in West Africa, outside of its previously reported and predicted niche. We assembled location data on all recorded zoonotic transmission to humans and Ebola virus infection in bats and primates (1976-2014). Using species distribution models, these occurrence data were paired with environmental covariates to predict a zoonotic transmission niche covering 22 countries across Central and West Africa. Vegetation, elevation, temperature, evapotranspiration, and suspected reservoir bat distributions define this relationship. At-risk areas are inhabited by 22 million people; however, the rarity of human outbreaks emphasises the very low probability of transmission to humans. Increasing population sizes and international connectivity by air since the first detection of EVD in 1976 suggest that the dynamics of human-to-human secondary transmission in contemporary outbreaks will be very different to those of the past.
Keywords: Ebola; Ebola virus; boosted regression trees; disease mapping; epidemiology; global health; human; infectious disease; microbiology; niche based modelling; species distribution modelling; viruses.
Conflict of interest statement
SIH: Reviewing editor,
The other authors declare that no competing interests exist.
Figures
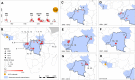
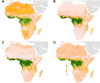


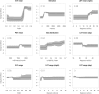

Comment in
-
Mapping Ebola in wild animals for better disease control.Elife. 2014 Sep 19;3:e04565. doi: 10.7554/eLife.04565. Elife. 2014. PMID: 25238569 Free PMC article.
References
-
- Baize S, Pannetier D, Oestereich L, Rieger T, Koivogui L, Magassouba N, Soropogui B, Sow MS, Keïta S, De Clerck H, Tiffany A, Dominguez G, Loua M, Traoré A, Kolié M, Malano ER, Heleze E, Bocquin A, Mély S, Raoul H, Caro V, Cadar D, Gabriel M, Pahlmann M, Tappe D, Schmidt-Chanasit J, Impouma B, Diallo AK, Formenty P, Van Herp M, Günther S. 2014. Emergence of Zaire Ebola virus disease in Guinea - preliminary report. The New England Journal of Medicine doi: 10.1056/NEJMoa1404505 - DOI - PubMed
-
- Barbet-Massin M, Jiguet F, Albert CH, Thuiller W. 2012. Selecting pseudo-absences for species distribution models: how, where and how many? Methods in Ecology and Evolution 3:327–338. doi: 10.1111/j.2041-210X.2011.00172.x - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

