Elimination of vitamin D receptor in vascular endothelial cells alters vascular function
- PMID: 25201890
- PMCID: PMC4430199
- DOI: 10.1161/HYPERTENSIONAHA.114.03971
Elimination of vitamin D receptor in vascular endothelial cells alters vascular function
Abstract
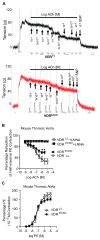
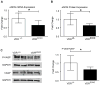
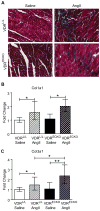
Vitamin D deficiency has been associated with cardiovascular dysfunction. We evaluated the role of the vitamin D receptor (VDR) in vascular endothelial function, a marker of cardiovascular health, at baseline and in the presence of angiotensin II, using an endothelial-specific knockout of the murine VDR gene. In the absence of endothelial VDR, acetylcholine-induced aortic relaxation was significantly impaired (maximal relaxation, endothelial-specific VDR knockout=58% versus control=73%; P<0.05). This was accompanied by a reduction in endothelial NO synthase expression and phospho-vasodilator-stimulated phosphoprotein levels in aortae from the endothelial-specific VDR knockout versus control mice. Although blood pressure levels at baseline were comparable at 12 and 24 weeks of age, the endothelial VDR knockout mice demonstrated increased sensitivity to the hypertensive effects of angiotensin II compared with control mice (after 1-week infusion: knockout=155±15 mm Hg versus control=133±7 mm Hg; P<0.01; after 2-week infusion: knockout=164±9 mm Hg versus control=152±13 mm Hg; P<0.05). By the end of 2 weeks, angiotensin II infusion-induced, hypertrophy-sensitive myocardial gene expression was higher in endothelial-specific VDR knockout mice (fold change compared with saline-infused control mice, type-A natriuretic peptide: knockout mice=3.12 versus control=1.7; P<0.05; type-B natriuretic peptide: knockout mice=4.72 versus control=2.68; P<0.05). These results suggest that endothelial VDR plays an important role in endothelial cell function and blood pressure control and imply a potential role for VDR agonists in the management of cardiovascular disease associated with endothelial dysfunction.
Keywords: gene targeting; hypertension; receptors, calcitriol; vitamin D.
© 2014 American Heart Association, Inc.
Figures




Comment in
-
Is at least one vitamin helping our vasculature? Evidence for an important role of the endothelial vitamin d receptor in regulating endothelial function and blood pressure.Hypertension. 2014 Dec;64(6):1187-8. doi: 10.1161/HYPERTENSIONAHA.114.04118. Epub 2014 Sep 8. Hypertension. 2014. PMID: 25201891 No abstract available.
References
-
- Holick MF. Vitamin d deficiency. N Engl J Med. 2007;357:266–281. - PubMed
-
- Haussler MR, Haussler CA, Bartik L, Whitfield GK, Hsieh J-C, Slater S, Jurutka PW. Vitamin d receptor: Molecular signaling and actions of nutritional ligands in disease prevention. Nutrition Reviews. 2008;66:S98–S112. - PubMed
-
- Forman JP, Giovannucci E, Holmes MD, Bischoff-Ferrari HA, Tworoger SS, Willett WC, Curhan GC. Plasma 25-hydroxyvitamin d levels and risk of incident hypertension. Hypertension. 2007;49:1063–1069. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

