Role of cavities and hydration in the pressure unfolding of T4 lysozyme
- PMID: 25201963
- PMCID: PMC4183293
- DOI: 10.1073/pnas.1410655111
Role of cavities and hydration in the pressure unfolding of T4 lysozyme
Abstract
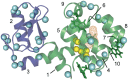
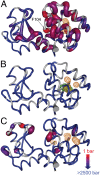
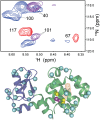
It is well known that high hydrostatic pressures can induce the unfolding of proteins. The physical underpinnings of this phenomenon have been investigated extensively but remain controversial. Changes in solvation energetics have been commonly proposed as a driving force for pressure-induced unfolding. Recently, the elimination of void volumes in the native folded state has been argued to be the principal determinant. Here we use the cavity-containing L99A mutant of T4 lysozyme to examine the pressure-induced destabilization of this multidomain protein by using solution NMR spectroscopy. The cavity-containing C-terminal domain completely unfolds at moderate pressures, whereas the N-terminal domain remains largely structured to pressures as high as 2.5 kbar. The sensitivity to pressure is suppressed by the binding of benzene to the hydrophobic cavity. These results contrast to the pseudo-WT protein, which has a residual cavity volume very similar to that of the L99A-benzene complex but shows extensive subglobal reorganizations with pressure. Encapsulation of the L99A mutant in the aqueous nanoscale core of a reverse micelle is used to examine the hydration of the hydrophobic cavity. The confined space effect of encapsulation suppresses the pressure-induced unfolding transition and allows observation of the filling of the cavity with water at elevated pressures. This indicates that hydration of the hydrophobic cavity is more energetically unfavorable than global unfolding. Overall, these observations point to a range of cooperativity and energetics within the T4 lysozyme molecule and illuminate the fact that small changes in physical parameters can significantly alter the pressure sensitivity of proteins.
Keywords: high-pressure NMR; protein folding and cooperativity; protein hydration; protein stability; reverse micelle encapsulation.
Conflict of interest statement
Conflict of interest statement: A.J.W. declares a competing financial interest as a member of Daedalus Innovations, LLC, a manufacturer of reverse micelle and high-pressure NMR apparatus.
Figures
Comment in
-
Is pressure-induced signal loss in NMR spectra for the Leu99Ala cavity mutant of T4 lysozyme due to unfolding?Proc Natl Acad Sci U S A. 2015 Mar 3;112(9):E923. doi: 10.1073/pnas.1423279112. Epub 2015 Jan 28. Proc Natl Acad Sci U S A. 2015. PMID: 25630507 Free PMC article. No abstract available.
-
Reply to Kitahara and Mulder: An ensemble view of protein stability best explains pressure effects in a T4 lysozyme cavity mutant.Proc Natl Acad Sci U S A. 2015 Mar 3;112(9):E924. doi: 10.1073/pnas.1424002112. Epub 2015 Jan 28. Proc Natl Acad Sci U S A. 2015. PMID: 25630509 Free PMC article. No abstract available.
References
-
- Zipp A, Kauzmann W. Pressure denaturation of metmyoglobin. Biochemistry. 1973;12(21):4217–4228. - PubMed
-
- Royer CA. Revisiting volume changes in pressure-induced protein unfolding. Biochim Biophys Acta. Protein Struct Mol Enzymol. 2002;1595:201–209. - PubMed
-
- Roche J, et al. Remodeling of the folding free energy landscape of staphylococcal nuclease by cavity-creating mutations. Biochemistry. 2012;51(47):9535–9546. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

