Motor coupling through lipid membranes enhances transport velocities for ensembles of myosin Va
- PMID: 25201964
- PMCID: PMC4183294
- DOI: 10.1073/pnas.1406535111
Motor coupling through lipid membranes enhances transport velocities for ensembles of myosin Va
Abstract
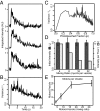
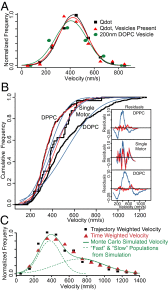
Myosin Va is an actin-based molecular motor responsible for transport and positioning of a wide array of intracellular cargoes. Although myosin Va motors have been well characterized at the single-molecule level, physiological transport is carried out by ensembles of motors. Studies that explore the behavior of ensembles of molecular motors have used nonphysiological cargoes such as DNA linkers or glass beads, which do not reproduce one key aspect of vesicular systems--the fluid intermotor coupling of biological lipid membranes. Using a system of defined synthetic lipid vesicles (100- to 650-nm diameter) composed of either 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) (fluid at room temperature) or 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) (gel at room temperature) with a range of surface densities of myosin Va motors (32-125 motors per μm(2)), we demonstrate that the velocity of vesicle transport by ensembles of myosin Va is sensitive to properties of the cargo. Gel-state DPPC vesicles bound with multiple motors travel at velocities equal to or less than vesicles with a single myosin Va (∼450 nm/s), whereas surprisingly, ensembles of myosin Va are able to transport fluid-state DOPC vesicles at velocities significantly faster (>700 nm/s) than a single motor. To explain these data, we developed a Monte Carlo simulation that suggests that these reductions in velocity can be attributed to two distinct mechanisms of intermotor interference (i.e., load-dependent modulation of stepping kinetics and binding-site exclusion), whereas faster transport velocities are consistent with a model wherein the normal stepping behavior of the myosin is supplemented by the preferential detachment of the trailing motor from the actin track.
Keywords: actin filament; liposome.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Cargo Transport by Two Coupled Myosin Va Motors on Actin Filaments and Bundles.Biophys J. 2016 Nov 15;111(10):2228-2240. doi: 10.1016/j.bpj.2016.09.046. Biophys J. 2016. PMID: 27851945 Free PMC article.
-
Small teams of myosin Vc motors coordinate their stepping for efficient cargo transport on actin bundles.J Biol Chem. 2017 Jun 30;292(26):10998-11008. doi: 10.1074/jbc.M117.780791. Epub 2017 May 5. J Biol Chem. 2017. PMID: 28476885 Free PMC article.
-
More than just a cargo adapter, melanophilin prolongs and slows processive runs of myosin Va.J Biol Chem. 2013 Oct 11;288(41):29313-22. doi: 10.1074/jbc.M113.476929. Epub 2013 Aug 26. J Biol Chem. 2013. PMID: 23979131 Free PMC article.
-
Vesicle transport: the role of actin filaments and myosin motors.Microsc Res Tech. 1999 Oct 15;47(2):93-106. doi: 10.1002/(SICI)1097-0029(19991015)47:2<93::AID-JEMT2>3.0.CO;2-P. Microsc Res Tech. 1999. PMID: 10523788 Review.
-
Cargo recognition and cargo-mediated regulation of unconventional myosins.Acc Chem Res. 2014 Oct 21;47(10):3061-70. doi: 10.1021/ar500216z. Epub 2014 Sep 17. Acc Chem Res. 2014. PMID: 25230296 Review.
Cited by
-
Diffusion of kinesin motors on cargo can enhance binding and run lengths during intracellular transport.Mol Biol Cell. 2021 Apr 19;32(9):984-994. doi: 10.1091/mbc.E20-10-0658. Epub 2021 Jan 13. Mol Biol Cell. 2021. PMID: 33439674 Free PMC article.
-
Cargo Transport by Two Coupled Myosin Va Motors on Actin Filaments and Bundles.Biophys J. 2016 Nov 15;111(10):2228-2240. doi: 10.1016/j.bpj.2016.09.046. Biophys J. 2016. PMID: 27851945 Free PMC article.
-
Membrane mediated motor kinetics in microtubule gliding assays.Sci Rep. 2019 Jul 3;9(1):9584. doi: 10.1038/s41598-019-45847-z. Sci Rep. 2019. PMID: 31270348 Free PMC article.
-
Microtubule Defects Influence Kinesin-Based Transport In Vitro.Biophys J. 2016 May 24;110(10):2229-40. doi: 10.1016/j.bpj.2016.04.029. Biophys J. 2016. PMID: 27224488 Free PMC article.
-
Mechanical coupling of microtubule-dependent motor teams during peroxisome transport in Drosophila S2 cells.Biochim Biophys Acta Gen Subj. 2017 Dec;1861(12):3178-3189. doi: 10.1016/j.bbagen.2017.09.009. Epub 2017 Sep 19. Biochim Biophys Acta Gen Subj. 2017. PMID: 28935608 Free PMC article.
References
-
- Desnos C, Huet S, Darchen F. “Should I stay or should I go?”: Myosin V function in organelle trafficking. Biol Cell. 2007;99(8):411–423. - PubMed
-
- Li JF, Nebenführ A. The tail that wags the dog: The globular tail domain defines the function of myosin V/XI. Traffic. 2008;9(3):290–298. - PubMed
-
- Tabb JS, Molyneaux BJ, Cohen DL, Kuznetsov SA, Langford GM. Transport of ER vesicles on actin filaments in neurons by myosin V. J Cell Sci. 1998;111(Pt 21):3221–3234. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

