Biophysical implications of lipid bilayer rheometry for mechanosensitive channels
- PMID: 25201991
- PMCID: PMC4183281
- DOI: 10.1073/pnas.1409011111
Biophysical implications of lipid bilayer rheometry for mechanosensitive channels
Abstract
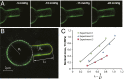
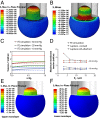
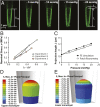
The lipid bilayer plays a crucial role in gating of mechanosensitive (MS) channels. Hence it is imperative to elucidate the rheological properties of lipid membranes. Herein we introduce a framework to characterize the mechanical properties of lipid bilayers by combining micropipette aspiration (MA) with theoretical modeling. Our results reveal that excised liposome patch fluorometry is superior to traditional cell-attached MA for measuring the intrinsic mechanical properties of lipid bilayers. The computational results also indicate that unlike the uniform bilayer tension estimated by Laplace's law, bilayer tension is not uniform across the membrane patch area. Instead, the highest tension is seen at the apex of the patch and the lowest tension is encountered near the pipette wall. More importantly, there is only a negligible difference between the stress profiles of the outer and inner monolayers in the cell-attached configuration, whereas a substantial difference (∼30%) is observed in the excised configuration. Our results have far-reaching consequences for the biophysical studies of MS channels and ion channels in general, using the patch-clamp technique, and begin to unravel the difference in activity seen between MS channels in different experimental paradigms.
Keywords: MscL; azolectin; electrophysiology; finite element modeling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Häse CC, Le Dain AC, Martinac B. Purification and functional reconstitution of the recombinant large mechanosensitive ion channel (MscL) of Escherichia coli. J Biol Chem. 1995;270(31):18329–18334. - PubMed
-
- Martinac B. Bacterial mechanosensitive channels as a paradigm for mechanosensory transduction. Cell Physiol Biochem. 2011;28(6):1051–1060. - PubMed
-
- Cox CD, et al. Selectivity mechanism of the mechanosensitive channel MscS revealed by probing channel subconducting states. Nat Commun. 2013;4:2137. - PubMed
-
- Maroto R, et al. TRPC1 forms the stretch-activated cation channel in vertebrate cells. Nat Cell Biol. 2005;7(2):179–185. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

