The LQLP calcineurin docking site is a major determinant of the calcium-dependent activation of human TRESK background K+ channel
- PMID: 25202008
- PMCID: PMC4207969
- DOI: 10.1074/jbc.M114.577684
The LQLP calcineurin docking site is a major determinant of the calcium-dependent activation of human TRESK background K+ channel
Abstract
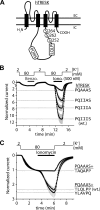
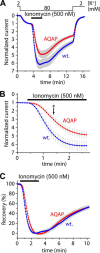
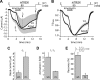
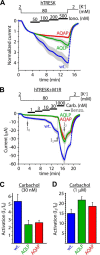
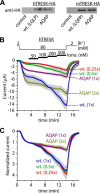
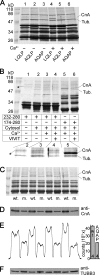
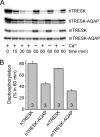
Calcium-dependent activation of human TRESK (TWIK-related spinal cord K(+) channel, K2P18.1) depends on direct targeting of calcineurin to the PQIIIS motif. In the present study we demonstrate that TRESK also contains another functionally relevant docking site for the phosphatase, the LQLP amino acid sequence. Combined mutations of the PQIIIS and LQLP motifs were required to eliminate the calcium-dependent regulation of the channel. In contrast to the alanine substitutions of PQIIIS, the mutation of LQLP to AQAP alone did not significantly change the amplitude of TRESK activation evoked by the substantial elevation of cytoplasmic calcium concentration. However, the AQAP mutation slowed down the response to high calcium. In addition, modest elevation of [Ca(2+)], which effectively regulated the wild type channel, failed to activate TRESK-AQAP. This indicates that the AQAP mutation diminished the sensitivity of TRESK to calcium. Even if PQIIIS was replaced by the PVIVIT sequence of high calcineurin binding affinity, the effect of the AQAP mutation was clearly detected in this TRESK-PVIVIT context. Substitution of the LQLP region with the corresponding fragment of NFAT transcription factor, perfectly matching the previously described LXVP calcineurin-binding consensus sequence, increased the calcium-sensitivity of TRESK-PVIVIT. Thus the enhancement of the affinity of TRESK for calcineurin by the incorporation of PVIVIT could not compensate for or prevent the effects of LQLP sequence modifications, suggesting that the two calcineurin-binding regions play distinct roles in the regulation. Our results indicate that the LQLP site is a fundamental determinant of the calcium-sensitivity of human TRESK.
Keywords: Calcineurin; Calcium; Ion Channel; K2P; KCNK18; PXIXIT; Potassium Channel; Protein Phosphatase 2B (PP2B); Two-pore Domain.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures

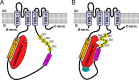
Similar articles
-
Properties, regulation, pharmacology, and functions of the K₂p channel, TRESK.Pflugers Arch. 2015 May;467(5):945-58. doi: 10.1007/s00424-014-1634-8. Epub 2014 Nov 5. Pflugers Arch. 2015. PMID: 25366493 Review.
-
Targeting of calcineurin to an NFAT-like docking site is required for the calcium-dependent activation of the background K+ channel, TRESK.J Biol Chem. 2006 May 26;281(21):14677-82. doi: 10.1074/jbc.M602495200. Epub 2006 Mar 28. J Biol Chem. 2006. PMID: 16569637
-
TRESK (K2P18.1) Background Potassium Channel Is Activated by Novel-Type Protein Kinase C via Dephosphorylation.Mol Pharmacol. 2019 Jun;95(6):661-672. doi: 10.1124/mol.119.116269. Epub 2019 Apr 16. Mol Pharmacol. 2019. PMID: 30992311
-
TRESK background K(+) channel is inhibited by phosphorylation via two distinct pathways.J Biol Chem. 2010 May 7;285(19):14549-57. doi: 10.1074/jbc.M110.102020. Epub 2010 Mar 9. J Biol Chem. 2010. PMID: 20215114 Free PMC article.
-
TRESK: the lone ranger of two-pore domain potassium channels.Mol Cell Endocrinol. 2012 Apr 28;353(1-2):75-81. doi: 10.1016/j.mce.2011.11.009. Epub 2011 Nov 17. Mol Cell Endocrinol. 2012. PMID: 22115960 Review.
Cited by
-
From phagocytosis to metaforosis: Calcineurin's deadly role in innate processing of fungi.PLoS Pathog. 2018 Jan 4;14(1):e1006627. doi: 10.1371/journal.ppat.1006627. eCollection 2018 Jan. PLoS Pathog. 2018. PMID: 29300778 Free PMC article. No abstract available.
-
Two-pore domain potassium channels: potential therapeutic targets for the treatment of pain.Pflugers Arch. 2015 May;467(5):931-43. doi: 10.1007/s00424-014-1655-3. Epub 2014 Nov 26. Pflugers Arch. 2015. PMID: 25420526 Review.
-
Properties, regulation, pharmacology, and functions of the K₂p channel, TRESK.Pflugers Arch. 2015 May;467(5):945-58. doi: 10.1007/s00424-014-1634-8. Epub 2014 Nov 5. Pflugers Arch. 2015. PMID: 25366493 Review.
-
Verapamil Inhibits TRESK (K2P18.1) Current in Trigeminal Ganglion Neurons Independently of the Blockade of Ca2+ Influx.Int J Mol Sci. 2018 Jul 4;19(7):1961. doi: 10.3390/ijms19071961. Int J Mol Sci. 2018. PMID: 29973548 Free PMC article.
-
KCNK18 Biallelic Variants Associated with Intellectual Disability and Neurodevelopmental Disorders Alter TRESK Channel Activity.Int J Mol Sci. 2021 Jun 4;22(11):6064. doi: 10.3390/ijms22116064. Int J Mol Sci. 2021. PMID: 34199759 Free PMC article.
References
-
- Enyedi P., Czirják G. (2010) Molecular background of leak K+ currents: two-pore domain potassium channels. Physiol. Rev. 90, 559–605 - PubMed
-
- Lotshaw D. P. (2007) Biophysical, pharmacological, and functional characteristics of cloned and native mammalian two-pore domain K+ channels. Cell Biochem. Biophys. 47, 209–256 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

