Cytoplasmic domain interactions of syndecan-1 and syndecan-4 with α6β4 integrin mediate human epidermal growth factor receptor (HER1 and HER2)-dependent motility and survival
- PMID: 25202019
- PMCID: PMC4215216
- DOI: 10.1074/jbc.M114.586438
Cytoplasmic domain interactions of syndecan-1 and syndecan-4 with α6β4 integrin mediate human epidermal growth factor receptor (HER1 and HER2)-dependent motility and survival
Abstract
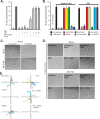
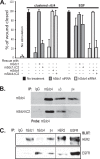
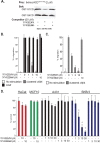
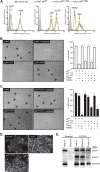
Epithelial cells are highly dependent during wound healing and tumorigenesis on the α6β4 integrin and its association with receptor tyrosine kinases. Previous work showed that phosphorylation of the β4 subunit upon matrix engagement depends on the matrix receptor syndecan (Sdc)-1 engaging the cytoplasmic domain of the β4 integrin and coupling of the integrin to human epidermal growth factor receptor-2 (HER2). In this study, HER2-dependent migration activated by matrix engagement is compared with migration stimulated by EGF. We find that whereas HER2-dependent migration depends on Sdc1, EGF-dependent migration depends on a complex consisting of human epidermal growth factor receptor-1 (HER1, commonly known as EGFR), α6β4, and Sdc4. The two syndecans recognize distinct sites at the extreme C terminus of the β4 integrin cytoplasmic domain. The binding motif in Sdc1 is QEEXYX, composed in part by its syndecan-specific variable (V) region and in part by the second conserved (C2) region that it shares with other syndecans. A cell-penetrating peptide containing this sequence competes for HER2-dependent epithelial migration and carcinoma survival, although it is without effect on the EGFR-stimulated mechanism. β4 mutants bearing mutations specific for Sdc1 and Sdc4 recognition act as dominant negative mutants to block cell spreading or cell migration that depends on HER2 or EGFR, respectively. The interaction of the α6β4 integrin with the syndecans appears critical for it to be utilized as a signaling platform; migration depends on α3β1 integrin binding to laminin 332 (LN332; also known as laminin 5), whereas antibodies that block α6β4 binding are without effect. These findings indicate that specific syndecan family members are likely to have key roles in α6β4 integrin activation by receptor tyrosine kinases.
Keywords: Carcinogenesis; Cell Invasion; Epidermal Growth Factor Receptor (EGFR); Integrin; Laminin; Syndecan; Wound Healing.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


References
-
- Aumailley M., Bruckner-Tuderman L., Carter W. G., Deutzmann R., Edgar D., Ekblom P., Engel J., Engvall E., Hohenester E., Jones J. C., Kleinman H. K., Marinkovich M. P., Martin G. R., Mayer U., Meneguzzi G., Miner J. H., Miyazaki K., Patarroyo M., Paulsson M., Quaranta V., Sanes J. R., Sasaki T., Sekiguchi K., Sorokin L. M., Talts J. F., Tryggvason K., Uitto J., Virtanen I., von der Mark K., Wewer U. M., Yamada Y., Yurchenco P. D. (2005) A simplified laminin nomenclature. Matrix Biol. 24, 326–332 - PubMed
-
- Nievers M. G., Schaapveld R. Q., Sonnenberg A. (1999) Biology and function of hemidesmosomes. Matrix Biol. 18, 5–17 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

