Transglutaminase II/microRNA-218/-181a loop regulates positive feedback relationship between allergic inflammation and tumor metastasis
- PMID: 25202021
- PMCID: PMC4207968
- DOI: 10.1074/jbc.M114.603480
Transglutaminase II/microRNA-218/-181a loop regulates positive feedback relationship between allergic inflammation and tumor metastasis
Abstract
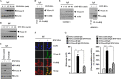
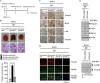
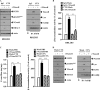
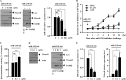
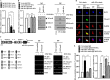
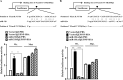
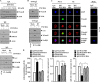
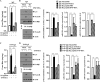
The molecular mechanism of transglutaminase II (TGaseII)-mediated allergic inflammation remains largely unknown. TGaseII, induced by antigen stimulation, showed an interaction and co-localization with FcϵRI. TGaseII was necessary for in vivo allergic inflammation, such as triphasic cutaneous reaction, passive cutaneous anaphylaxis, and passive systemic anaphylaxis. TGaseII was necessary for the enhanced metastatic potential of B16F1 melanoma cells by passive systemic anaphylaxis. TGaseII was shown to be a secreted protein. Recombinant TGaseII protein increased the histamine release and β-hexosaminidase activity, and enhanced the metastatic potential of B16F1 mouse melanoma cells. Recombinant TGaseII protein induced the activation of EGF receptor and an interaction between EGF receptor and FcϵRI. Recombinant TGaseII protein displayed angiogenic potential accompanied by allergic inflammation. R2 peptide, an inhibitor of TGaseII, exerted negative effects on in vitro and in vivo allergic inflammation by regulating the expression of TGaseII and FcϵRI signaling. MicroRNA (miR)-218 and miR-181a, decreased during allergic inflammation, were predicted as negative regulators of TGaseII by microRNA array and TargetScan analysis. miR-218 and miR-181a formed a negative feedback loop with TGaseII and regulated the in vitro and in vivo allergic inflammation. TGaseII was necessary for the interaction between mast cells and macrophages during allergic inflammation. Mast cells and macrophages, activated during allergic inflammation, were responsible for the enhanced metastatic potential of tumor cells that are accompanied by allergic inflammation. In conclusion, the TGaseII/miR-218/-181a feedback loop can be employed for the development of anti-allergy therapeutics.
Keywords: Allergic Inflammation; Angiogenesis; Gene Regulation; Macrophage; Mast Cell; MicroRNA-181a; MicroRNA-218; Transglutaminase II; Tumor Metastasis.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures









Similar articles
-
MicroRNA-26a/-26b-COX-2-MIP-2 Loop Regulates Allergic Inflammation and Allergic Inflammation-promoted Enhanced Tumorigenic and Metastatic Potential of Cancer Cells.J Biol Chem. 2015 May 29;290(22):14245-66. doi: 10.1074/jbc.M115.645580. Epub 2015 Apr 23. J Biol Chem. 2015. PMID: 25907560 Free PMC article.
-
Histone deacetylase-3 mediates positive feedback relationship between anaphylaxis and tumor metastasis.J Biol Chem. 2014 Apr 25;289(17):12126-12144. doi: 10.1074/jbc.M113.521245. Epub 2014 Mar 11. J Biol Chem. 2014. PMID: 24619412 Free PMC article.
-
MiR-135-5p-p62 Axis Regulates Autophagic Flux, Tumorigenic Potential, and Cellular Interactions Mediated by Extracellular Vesicles During Allergic Inflammation.Front Immunol. 2019 Apr 5;10:738. doi: 10.3389/fimmu.2019.00738. eCollection 2019. Front Immunol. 2019. PMID: 31024564 Free PMC article.
-
MiR-154-5p-MCP1 Axis Regulates Allergic Inflammation by Mediating Cellular Interactions.Front Immunol. 2021 May 31;12:663726. doi: 10.3389/fimmu.2021.663726. eCollection 2021. Front Immunol. 2021. PMID: 34135893 Free PMC article.
-
[Role of Histamine-releasing Factor in Allergic Inflammatory Reactions].Yakugaku Zasshi. 2017;137(5):517-521. doi: 10.1248/yakushi.16-00239-3. Yakugaku Zasshi. 2017. PMID: 28458281 Review. Japanese.
Cited by
-
Human Adipose Tissue-Derived Mesenchymal Stem Cells Attenuate Atopic Dermatitis by Regulating the Expression of MIP-2, miR-122a-SOCS1 Axis, and Th1/Th2 Responses.Front Pharmacol. 2018 Nov 6;9:1175. doi: 10.3389/fphar.2018.01175. eCollection 2018. Front Pharmacol. 2018. PMID: 30459600 Free PMC article.
-
Piceatannol induced apoptosis through up-regulation of microRNA-181a in melanoma cells.Biol Res. 2017 Oct 17;50(1):36. doi: 10.1186/s40659-017-0141-8. Biol Res. 2017. PMID: 29041990 Free PMC article.
-
miR-122-SOCS1-JAK2 axis regulates allergic inflammation and allergic inflammation-promoted cellular interactions.Oncotarget. 2017 Jul 10;8(38):63155-63176. doi: 10.18632/oncotarget.19149. eCollection 2017 Sep 8. Oncotarget. 2017. PMID: 28968979 Free PMC article.
-
MicroRNA-365 inhibits growth, invasion and metastasis of malignant melanoma by targeting NRP1 expression.Int J Clin Exp Pathol. 2015 May 1;8(5):4913-22. eCollection 2015. Int J Clin Exp Pathol. 2015. PMID: 26191184 Free PMC article.
-
Regulation of allergic lung inflammation by endothelial cell transglutaminase 2.Am J Physiol Lung Cell Mol Physiol. 2015 Sep 15;309(6):L573-83. doi: 10.1152/ajplung.00199.2015. Epub 2015 Jul 24. Am J Physiol Lung Cell Mol Physiol. 2015. PMID: 26209276 Free PMC article.
References
-
- Fesus L., Szucs E. F., Barrett K. E., Metcalfe D. D., Folk J. E. (1985) Activation of transglutaminase and production of protein-bound γ-glutamylhistamine in stimulated mouse mast cells. J. Biol. Chem. 260, 13771–13778 - PubMed
-
- Palosuo K., Varjonen E., Nurkkala J., Kalkkinen N., Harvima R., Reunala T., Alenius H. (2003) Transglutaminase-mediated cross-linking of a peptic fraction of ω-5 gliadin enhances IgE reactivity in wheat-dependent, exercise-induced anaphylaxis. J. Allergy Clin. Immunol. 111, 1386–1392 - PubMed
-
- Lai T. S., Greenberg C. S. (2013) Histaminylation of fibrinogen by tissue transglutaminase-2 (TGM-2): potential role in modulating inflammation. Amino Acids 45, 857–864 - PubMed
-
- Molberg O., Mcadam S. N., Körner R., Quarsten H., Kristiansen C., Madsen L., Fugger L., Scott H., Norén O., Roepstorff P., Lundin K. E., Sjöström H., Sollid L. M. (1998) Tissue transglutaminase selectively modifies gliadin peptides that are recognized by gut-derived T cells in celiac disease. Nat. Med. 4, 713–717 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

