C-type lectin receptor dectin-3 mediates trehalose 6,6'-dimycolate (TDM)-induced Mincle expression through CARD9/Bcl10/MALT1-dependent nuclear factor (NF)-κB activation
- PMID: 25202022
- PMCID: PMC4208012
- DOI: 10.1074/jbc.M114.588574
C-type lectin receptor dectin-3 mediates trehalose 6,6'-dimycolate (TDM)-induced Mincle expression through CARD9/Bcl10/MALT1-dependent nuclear factor (NF)-κB activation
Abstract
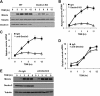
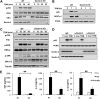
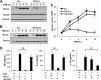
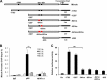
Previous studies indicate that both Dectin-3 (also called MCL or Clec4d) and Mincle (also called Clec4e), two C-type lectin receptors, can recognize trehalose 6,6'-dimycolate (TDM), a cell wall component from mycobacteria, and induce potent innate immune responses. Interestingly, stimulation of Dectin-3 by TDM can also induce Mincle expression, which may enhance the host innate immune system to sense Mycobacterium infection. However, the mechanism by which Dectin-3 induces Mincle expression is not fully defined. Here, we show that TDM-induced Mincle expression is dependent on Dectin-3-mediated NF-κB, but not nuclear factor of activated T-cells (NFAT), activation, and Dectin-3 induces NF-κB activation through the CARD9-BCL10-MALT1 complex. We found that bone marrow-derived macrophages from Dectin-3-deficient mice were severely defective in the induction of Mincle expression in response to TDM stimulation. This defect is correlated with the failure of TDM-induced NF-κB activation in Dectin-3-deficient bone marrow-derived macrophages. Consistently, inhibition of NF-κB, but not NFAT, impaired TDM-induced Mincle expression, whereas NF-κB, but not NFAT, binds to the Mincle promoter. Dectin-3-mediated NF-κB activation is dependent on the CARD9-Bcl10-MALT1 complex. Finally, mice deficient for Dectin-3 or CARD9 produced much less proinflammatory cytokines and keyhole limpet hemocyanin (KLH)-specific antibodies after immunization with an adjuvant containing TDM. Overall, this study provides the mechanism by which Dectin-3 induces Mincle expression in response to Mycobacterium infection, which will have significant impact to improve adjuvant and design vaccine for antimicrobial infection.
Keywords: Innate Immunity; Lectin; NF-kappa B (NF-κB); NF-κB Transcription Factor; Signal Transduction.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures

Similar articles
-
Phospholipase Cgamma2 is critical for Dectin-1-mediated Ca2+ flux and cytokine production in dendritic cells.J Biol Chem. 2009 Mar 13;284(11):7038-46. doi: 10.1074/jbc.M806650200. Epub 2009 Jan 9. J Biol Chem. 2009. PMID: 19136564 Free PMC article.
-
Contribution of MINCLE-SYK Signaling to Activation of Primary Human APCs by Mycobacterial Cord Factor and the Novel Adjuvant TDB.J Immunol. 2015 Sep 1;195(5):2417-28. doi: 10.4049/jimmunol.1500102. Epub 2015 Jul 22. J Immunol. 2015. PMID: 26202982
-
Adaptor protein 3BP2 regulates gene expression in addition to the ubiquitination and proteolytic activity of MALT1 in dectin-1-stimulated cells.J Biol Chem. 2024 Dec;300(12):107980. doi: 10.1016/j.jbc.2024.107980. Epub 2024 Nov 13. J Biol Chem. 2024. PMID: 39542253 Free PMC article.
-
Genetic errors of the human caspase recruitment domain-B-cell lymphoma 10-mucosa-associated lymphoid tissue lymphoma-translocation gene 1 (CBM) complex: Molecular, immunologic, and clinical heterogeneity.J Allergy Clin Immunol. 2015 Nov;136(5):1139-49. doi: 10.1016/j.jaci.2015.06.031. Epub 2015 Aug 12. J Allergy Clin Immunol. 2015. PMID: 26277595 Free PMC article. Review.
-
Role of the CARMA1/BCL10/MALT1 complex in lymphoid malignancies.Curr Opin Hematol. 2016 Jul;23(4):402-9. doi: 10.1097/MOH.0000000000000257. Curr Opin Hematol. 2016. PMID: 27135977 Free PMC article. Review.
Cited by
-
Activation and Inhibition of the NLRP3 Inflammasome by RNA Viruses.J Inflamm Res. 2021 Mar 26;14:1145-1163. doi: 10.2147/JIR.S295706. eCollection 2021. J Inflamm Res. 2021. PMID: 33814921 Free PMC article. Review.
-
Recent insights into structures and functions of C-type lectins in the immune system.Curr Opin Struct Biol. 2015 Oct;34:26-34. doi: 10.1016/j.sbi.2015.06.003. Epub 2015 Jul 7. Curr Opin Struct Biol. 2015. PMID: 26163333 Free PMC article. Review.
-
E3 ubiquitin ligase Cbl-b negatively regulates C-type lectin receptor-mediated antifungal innate immunity.J Exp Med. 2016 Jul 25;213(8):1555-70. doi: 10.1084/jem.20151932. Epub 2016 Jul 18. J Exp Med. 2016. PMID: 27432944 Free PMC article.
-
CARD9 Mediates Pancreatic Islet Beta-Cell Dysfunction Under the Duress of Hyperglycemic Stress.Cell Physiol Biochem. 2022 Apr 1;56(2):120-137. doi: 10.33594/000000508. Cell Physiol Biochem. 2022. PMID: 35362297 Free PMC article.
-
Immune Interactions with Pathogenic and Commensal Fungi: A Two-Way Street.Immunity. 2015 Nov 17;43(5):845-58. doi: 10.1016/j.immuni.2015.10.023. Immunity. 2015. PMID: 26588778 Free PMC article. Review.
References
-
- Netea M. G., Brown G. D., Kullberg B. J., Gow N. A. (2008) An integrated model of the recognition of Candida albicans by the innate immune system. Nat. Rev. Microbiol. 6, 67–78 - PubMed
-
- Vautier S., MacCallum D. M., Brown G. D. (2012) C-type lectin receptors and cytokines in fungal immunity. Cytokine 58, 89–99 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

