Antiepileptic drug treatment of rolandic epilepsy and Panayiotopoulos syndrome: clinical practice survey and clinical trial feasibility
- PMID: 25202134
- PMCID: PMC4283698
- DOI: 10.1136/archdischild-2013-304211
Antiepileptic drug treatment of rolandic epilepsy and Panayiotopoulos syndrome: clinical practice survey and clinical trial feasibility
Abstract
Background: The evidence base for management of childhood epilepsy is poor, especially for the most common specific syndromes such as rolandic epilepsy (RE) and Panayiotopoulos syndrome (PS). Considerable international variation in management and controversy about non-treatment indicate the need for high quality randomised controlled trials (RCT). The aim of this study is, therefore, to describe current UK practice and explore the feasibility of different RCT designs for RE and PS.
Methods: We conducted an online survey of 590 UK paediatricians who treat epilepsy. Thirty-two questions covered annual caseload, investigation and management practice, factors influencing treatment, antiepileptic drug preferences and hypothetical trial design preferences.
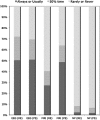
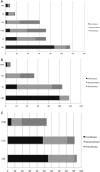
Results: 132 responded (22%): 81% were paediatricians and 95% at consultant seniority. We estimated, annually, 751 new RE cases and 233 PS cases. Electroencephalography (EEG) is requested at least half the time in approximately 70% of cases; MRI brain at least half the time in 40%-65% cases and neuropsychological evaluation in 7%-8%. Clinicians reported non-treatment in 40%: main reasons were low frequency of seizures and parent/child preferences. Carbamazepine is the preferred older, and levetiracetam the preferred newer, RCT arm. Approximately one-half considered active and placebo designs acceptable, choosing seizures as primary and cognitive/behavioural measures as secondary outcomes.
Conclusions: Management among respondents is broadly in line with national guidance, although with possible overuse of brain imaging and underuse of EEG and neuropsychological assessments. A large proportion of patients in the UK remains untreated, and clinicians seem amenable to a range of RCT designs, with carbamazepine and levetiracetam the preferred active drugs.
Keywords: Neurology; Paediatric Practice.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Figures

References
-
- Joint Epilepsy Council. Epilepsy prevalence, incidence and other statistics. www.jointepilepsycouncil.org.uk; Leeds 2011.
-
- Camfield C, Breau L, Camfield P. Assessing the impact of pediatric epilepsy and concomitant behavioral, cognitive, and physical/neurologic disability: impact of childhood neurologic disability scale. Dev Med Child Neurol 2003;45:152–9. - PubMed
-
- Institute of Medicine (US). Committee on the public health dimensions of the epilepsies board on health sciences policy. In: England MJ, Liverman CT, Schultz AM, Strawbridge LM. eds. Epilepsy across the spectrum. Promoting health and understanding. Washington, DC: Institute of Medicine of the National Academies, 2012:572. - PubMed
-
- Connolly AM, Northcott E, Cairns DR, et al. Quality of life of children with benign rolandic epilepsy. Pediatr Neurol 2006;35:240–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
