The engine of the reef: photobiology of the coral-algal symbiosis
- PMID: 25202301
- PMCID: PMC4141621
- DOI: 10.3389/fmicb.2014.00422
The engine of the reef: photobiology of the coral-algal symbiosis
Abstract
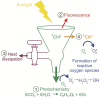
Coral reef ecosystems thrive in tropical oligotrophic oceans because of the relationship between corals and endosymbiotic dinoflagellate algae called Symbiodinium. Symbiodinium convert sunlight and carbon dioxide into organic carbon and oxygen to fuel coral growth and calcification, creating habitat for these diverse and productive ecosystems. Light is thus a key regulating factor shaping the productivity, physiology, and ecology of the coral holobiont. Similar to all oxygenic photoautotrophs, Symbiodinium must safely harvest sunlight for photosynthesis and dissipate excess energy to prevent oxidative stress. Oxidative stress is caused by environmental stressors such as those associated with global climate change, and ultimately leads to breakdown of the coral-algal symbiosis known as coral bleaching. Recently, large-scale coral bleaching events have become pervasive and frequent threatening and endangering coral reefs. Because the coral-algal symbiosis is the biological engine producing the reef, the future of coral reef ecosystems depends on the ecophysiology of the symbiosis. This review examines the photobiology of the coral-algal symbiosis with particular focus on the photophysiological responses and timescales of corals and Symbiodinium. Additionally, this review summarizes the light environment and its dynamics, the vulnerability of the symbiosis to oxidative stress, the abiotic and biotic factors influencing photosynthesis, the diversity of the coral-algal symbiosis, and recent advances in the field. Studies integrating physiology with the developing "omics" fields will provide new insights into the coral-algal symbiosis. Greater physiological and ecological understanding of the coral-algal symbiosis is needed for protection and conservation of coral reefs.
Keywords: Symbiodinium; acclimation; dinoflagellate; ecophysiology; photophysiology; photoprotection; scleractinian corals.
Figures


Similar articles
-
Change in algal symbiont communities after bleaching, not prior heat exposure, increases heat tolerance of reef corals.Glob Chang Biol. 2015 Jan;21(1):236-49. doi: 10.1111/gcb.12706. Epub 2014 Sep 9. Glob Chang Biol. 2015. PMID: 25099991
-
Moderate Thermal Stress Causes Active and Immediate Expulsion of Photosynthetically Damaged Zooxanthellae (Symbiodinium) from Corals.PLoS One. 2014 Dec 10;9(12):e114321. doi: 10.1371/journal.pone.0114321. eCollection 2014. PLoS One. 2014. PMID: 25493938 Free PMC article.
-
Rapid thermal adaptation in photosymbionts of reef-building corals.Glob Chang Biol. 2017 Nov;23(11):4675-4688. doi: 10.1111/gcb.13702. Epub 2017 Apr 27. Glob Chang Biol. 2017. PMID: 28447372
-
Coral photobiology: new light on old views.Zoology (Jena). 2015 Apr;118(2):71-8. doi: 10.1016/j.zool.2014.08.003. Epub 2014 Nov 3. Zoology (Jena). 2015. PMID: 25467066 Review.
-
The trace metal economy of the coral holobiont: supplies, demands and exchanges.Biol Rev Camb Philos Soc. 2023 Apr;98(2):623-642. doi: 10.1111/brv.12922. Epub 2022 Nov 22. Biol Rev Camb Philos Soc. 2023. PMID: 36897260 Review.
Cited by
-
Measuring light scattering and absorption in corals with Inverse Spectroscopic Optical Coherence Tomography (ISOCT): a new tool for non-invasive monitoring.Sci Rep. 2019 Oct 2;9(1):14148. doi: 10.1038/s41598-019-50658-3. Sci Rep. 2019. PMID: 31578438 Free PMC article.
-
Genome-wide analysis to uncover how Pocillopora acuta survives the challenging intertidal environment.Sci Rep. 2024 Apr 12;14(1):8538. doi: 10.1038/s41598-024-59268-0. Sci Rep. 2024. PMID: 38609456 Free PMC article.
-
Ocean acidification and nitrate enrichment can mitigate negative effects of soft coral (Xenia) competition on hard coral (Stylophora pistillata) endosymbionts.Sci Rep. 2025 Aug 15;15(1):29937. doi: 10.1038/s41598-025-15683-5. Sci Rep. 2025. PMID: 40817345 Free PMC article.
-
Acute Toxicity Assays with Adult Coral Fragments: A Method for Standardization.Toxics. 2023 Dec 19;12(1):1. doi: 10.3390/toxics12010001. Toxics. 2023. PMID: 38276714 Free PMC article.
-
Assessment of the recovery and photosynthetic efficiency of Breviolum psygmophilum and Effrenium voratum (Symbiodiniaceae) following cryopreservation.PeerJ. 2023 Feb 28;11:e14885. doi: 10.7717/peerj.14885. eCollection 2023. PeerJ. 2023. PMID: 36874975 Free PMC article.
References
-
- Allemand D., Tambutté E., Zoccola D., Tambutté S. (2011). “Coral calcification, cells to reefs,” in Coral Reefs: An Ecosystem in Transition eds Dubinsky Z., Stambler N. (Amsterdam: Springer; ) 119–150
-
- Anthony K. R. N., Hoegh-Guldberg O. (2003b). Variation in coral photosynthesis, respiration and growth characteristics in contrasting light microhabitats: an analogue to plants in forest gaps and understoreys? Funct. Ecol. 17 246–259 10.2307/3599181 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

