Differential site accessibility mechanistically explains subcellular-specific N-glycosylation determinants
- PMID: 25202310
- PMCID: PMC4142333
- DOI: 10.3389/fimmu.2014.00404
Differential site accessibility mechanistically explains subcellular-specific N-glycosylation determinants
Abstract
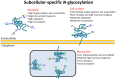
Glycoproteins perform extra- and intracellular functions in innate and adaptive immunity by lectin-based interactions to exposed glyco-determinants. Herein, we document and mechanistically explain the formation of subcellular-specific N-glycosylation determinants on glycoproteins trafficking through the shared biosynthetic machinery of human cells. LC-MS/MS-based quantitative glycomics showed that the secreted glycoproteins of eight human breast epithelial cells displaying diverse geno- and phenotypes consistently displayed more processed, primarily complex type, N-glycans than the high-mannose-rich microsomal glycoproteins. Detailed subcellular glycome profiling of proteins derived from three breast cell lines (MCF7/MDA468/MCF10A) demonstrated that secreted glycoproteins displayed significantly more α-sialylation and α1,6-fucosylation, but less α-mannosylation, than both the intermediately glycan-processed cell-surface glycoproteomes and the under-processed microsomal glycoproteomes. Subcellular proteomics and gene ontology revealed substantial presence of endoplasmic reticulum resident glycoproteins in the microsomes and confirmed significant enrichment of secreted and cell-surface glycoproteins in the respective subcellular fractions. The solvent accessibility of the glycosylation sites on maturely folded proteins of the 100 most abundant putative N-glycoproteins observed uniquely in the three subcellular glycoproteomes correlated with the glycan type processing thereby mechanistically explaining the formation of subcellular-specific N-glycosylation. In conclusion, human cells have developed mechanisms to simultaneously and reproducibly generate subcellular-specific N-glycosylation using a shared biosynthetic machinery. This aspect of protein-specific glycosylation is important for structural and functional glycobiology and discussed here in the context of the spatio-temporal interaction of glyco-determinants with lectins central to infection and immunity.
Keywords: N-glycan; N-glycome; N-glycosylation; glycoprotein; glycoproteome; glycosylation site; solvent accessibility; subcellular location.
Figures

Similar articles
-
Mycobacterium tuberculosis Infection Manipulates the Glycosylation Machinery and the N-Glycoproteome of Human Macrophages and Their Microparticles.J Proteome Res. 2017 Jan 6;16(1):247-263. doi: 10.1021/acs.jproteome.6b00685. Epub 2016 Nov 4. J Proteome Res. 2017. PMID: 27760463
-
N-glycan occupancy of Arabidopsis N-glycoproteins.J Proteomics. 2013 Nov 20;93:343-55. doi: 10.1016/j.jprot.2013.07.032. Epub 2013 Aug 27. J Proteomics. 2013. PMID: 23994444
-
Site-specific glycoproteomics confirms that protein structure dictates formation of N-glycan type, core fucosylation and branching.Glycobiology. 2012 Nov;22(11):1440-52. doi: 10.1093/glycob/cws110. Epub 2012 Jul 13. Glycobiology. 2012. PMID: 22798492
-
Advances in LC-MS/MS-based glycoproteomics: getting closer to system-wide site-specific mapping of the N- and O-glycoproteome.Biochim Biophys Acta. 2014 Sep;1844(9):1437-52. doi: 10.1016/j.bbapap.2014.05.002. Epub 2014 May 12. Biochim Biophys Acta. 2014. PMID: 24830338 Review.
-
Human protein paucimannosylation: cues from the eukaryotic kingdoms.Biol Rev Camb Philos Soc. 2019 Dec;94(6):2068-2100. doi: 10.1111/brv.12548. Epub 2019 Aug 14. Biol Rev Camb Philos Soc. 2019. PMID: 31410980 Review.
Cited by
-
Reference glycan structure libraries of primary human cardiomyocytes and pluripotent stem cell-derived cardiomyocytes reveal cell-type and culture stage-specific glycan phenotypes.J Mol Cell Cardiol. 2020 Feb;139:33-46. doi: 10.1016/j.yjmcc.2019.12.012. Epub 2020 Jan 21. J Mol Cell Cardiol. 2020. PMID: 31972267 Free PMC article.
-
Carbohydrates in Cyberspace.Front Immunol. 2015 Jun 10;6:300. doi: 10.3389/fimmu.2015.00300. eCollection 2015. Front Immunol. 2015. PMID: 26113848 Free PMC article. Review. No abstract available.
-
Tissue-Specific Glycosylation at the Glycopeptide Level.Mol Cell Proteomics. 2015 Aug;14(8):2103-10. doi: 10.1074/mcp.M115.050393. Epub 2015 May 20. Mol Cell Proteomics. 2015. PMID: 25995273 Free PMC article.
-
Site-Specific N-Glycosylation of Recombinant Pentameric and Hexameric Human IgM.J Am Soc Mass Spectrom. 2016 Jul;27(7):1143-55. doi: 10.1007/s13361-016-1378-0. Epub 2016 Apr 1. J Am Soc Mass Spectrom. 2016. PMID: 27038031
-
Asn347 Glycosylation of Corticosteroid-binding Globulin Fine-tunes the Host Immune Response by Modulating Proteolysis by Pseudomonas aeruginosa and Neutrophil Elastase.J Biol Chem. 2016 Aug 19;291(34):17727-42. doi: 10.1074/jbc.M116.735258. Epub 2016 Jun 23. J Biol Chem. 2016. PMID: 27339896 Free PMC article.
References
-
- Cylwik B, Naklicki M, Chrostek L, Gruszewska E. Congenital disorders of glycosylation. Part I. Defects of protein N-glycosylation. Acta Biochim Pol (2013) 60(2):151–61 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

