Peptide inhibitor of complement c1, a novel suppressor of classical pathway activation: mechanistic studies and clinical potential
- PMID: 25202312
- PMCID: PMC4141160
- DOI: 10.3389/fimmu.2014.00406
Peptide inhibitor of complement c1, a novel suppressor of classical pathway activation: mechanistic studies and clinical potential
Abstract
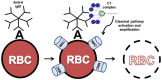
The classical pathway of complement plays multiple physiological roles including modulating immunological effectors initiated by adaptive immune responses and an essential homeostatic role in the clearance of damaged self-antigens. However, dysregulated classical pathway activation is associated with antibody-initiated, inflammatory diseases processes like cold agglutinin disease, acute intravascular hemolytic transfusion reaction (AIHTR), and acute/hyperacute transplantation rejection. To date, only one putative classical pathway inhibitor, C1 esterase inhibitor (C1-INH), is currently commercially available and its only approved indication is for replacement treatment in hereditary angioedema, which is predominantly a kinin pathway disease. Given the variety of disease conditions in which the classical pathway is implicated, development of therapeutics that specifically inhibits complement initiation represents a major unmet medical need. Our laboratory has identified a peptide that specifically inhibits the classical and lectin pathways of complement. In vitro studies have demonstrated that these peptide inhibitors of complement C1 (PIC1) bind to the collagen-like region of the initiator molecule of the classical pathway, C1q. PIC1 binding to C1q blocks activation of the associated serine proteases (C1s-C1r-C1r-C1s) and subsequent downstream complement activation. Rational design optimization of PIC1 has resulted in the generation of a highly potent derivative of 15 amino acids. PIC1 inhibits classical pathway mediated complement activation in ABO incompatibility in vitro and inhibiting classical pathway activation in vivo in rats. This review will focus on the pre-clinical development of PIC1 and discuss its potential as a therapeutic in antibody-mediated classical pathway disease, specifically AIHTR.
Keywords: ABO incompatibility; AIHTR; C1q; MBL; classical pathway; complement; inhibitor; peptide.
Figures
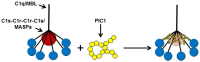
Similar articles
-
Peptide Inhibitor of Complement C1 (PIC1) Rapidly Inhibits Complement Activation after Intravascular Injection in Rats.PLoS One. 2015 Jul 21;10(7):e0132446. doi: 10.1371/journal.pone.0132446. eCollection 2015. PLoS One. 2015. PMID: 26196285 Free PMC article.
-
Human astrovirus coat protein binds C1q and MBL and inhibits the classical and lectin pathways of complement activation.Mol Immunol. 2010 Jan;47(4):792-8. doi: 10.1016/j.molimm.2009.10.006. Epub 2009 Nov 6. Mol Immunol. 2010. PMID: 19896716
-
Antibody-independent activation of C1. II. Evidence for two classes of nonimmune activators of the classical pathway of complement.J Immunol. 1987 Mar 15;138(6):1871-6. J Immunol. 1987. PMID: 3029223
-
Structure and function of complement activating enzyme complexes: C1 and MBL-MASPs.Curr Protein Pept Sci. 2001 Mar;2(1):43-59. doi: 10.2174/1389203013381242. Curr Protein Pept Sci. 2001. PMID: 12369900 Review.
-
Structure and function of C1 inhibitor.Behring Inst Mitt. 1989 Jul;(84):142-50. Behring Inst Mitt. 1989. PMID: 2679529 Review.
Cited by
-
Peptide Inhibitor of Complement C1 (PIC1) Rapidly Inhibits Complement Activation after Intravascular Injection in Rats.PLoS One. 2015 Jul 21;10(7):e0132446. doi: 10.1371/journal.pone.0132446. eCollection 2015. PLoS One. 2015. PMID: 26196285 Free PMC article.
-
The EPICC Family of Anti-Inflammatory Peptides: Next Generation Peptides, Additional Mechanisms of Action, and In Vivo and Ex Vivo Efficacy.Front Immunol. 2022 Feb 9;13:752315. doi: 10.3389/fimmu.2022.752315. eCollection 2022. Front Immunol. 2022. PMID: 35222367 Free PMC article. Review.
-
Peptide Inhibitor of Complement C1 (PIC1) demonstrates antioxidant activity via single electron transport (SET) and hydrogen atom transfer (HAT).PLoS One. 2018 Mar 2;13(3):e0193931. doi: 10.1371/journal.pone.0193931. eCollection 2018. PLoS One. 2018. PMID: 29499069 Free PMC article.
-
RLS-0071 Moderated Elevated Myeloperoxidase Level and Activity in an Asymptomatic Subject in Clinical Trial RLS-0071-101.Am J Case Rep. 2023 May 26;24:e939803. doi: 10.12659/AJCR.939803. Am J Case Rep. 2023. PMID: 37231631 Free PMC article.
-
Novel Evasion Mechanisms of the Classical Complement Pathway.J Immunol. 2016 Sep 15;197(6):2051-60. doi: 10.4049/jimmunol.1600863. J Immunol. 2016. PMID: 27591336 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

