Intraovarian control of early folliculogenesis
- PMID: 25202833
- PMCID: PMC4309737
- DOI: 10.1210/er.2014-1020
Intraovarian control of early folliculogenesis
Abstract
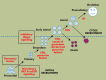
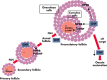
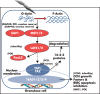
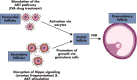
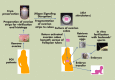
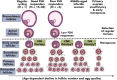
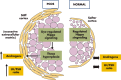
Although hormonal regulation of ovarian follicle development has been extensively investigated, most studies concentrate on the development of early antral follicles to the preovulatory stage, leading to the successful use of exogenous FSH for infertility treatment. Accumulating data indicate that preantral follicles are under stringent regulation by FSH and local intraovarian factors, thus providing the possibility to develop new therapeutic approaches. Granulosa cell-derived C-type natriuretic factor not only suppresses the final maturation of oocytes to undergo germinal vesicle breakdown before ovulation but also promotes preantral and antral follicle growth. In addition, several oocyte- and granulosa cell-derived factors stimulate preantral follicle growth by acting through wingless, receptor tyrosine kinase, receptor serine kinase, and other signaling pathways. In contrast, the ovarian Hippo signaling pathway constrains follicle growth and disruption of Hippo signaling promotes the secretion of downstream CCN growth factors capable of promoting follicle growth. Although the exact hormonal factors involved in primordial follicle activation has yet to be elucidated, the protein kinase B (AKT) and mammalian target of rapamycin signaling pathways are important for the activation of dormant primordial follicles. Hippo signaling disruption after ovarian fragmentation, combined with treating ovarian fragments with phosphatase and tensin homolog (PTEN) inhibitors and phosphoinositide-3-kinase stimulators to augment AKT signaling, promote the growth of preantral follicles in patients with primary ovarian insufficiency, leading to a new infertility intervention for such patients. Elucidation of intraovarian mechanisms underlying early folliculogenesis may allow the development of novel therapeutic strategies for patients diagnosed with primary ovarian insufficiency, polycystic ovary syndrome, and poor ovarian response to FSH stimulation, as well as for infertile women of advanced reproductive age.
Figures

References
-
- McGee EA, Hsueh AJ. Initial and cyclic recruitment of ovarian follicles. Endocr Rev. 2000;21:200–214. - PubMed
-
- Hsueh AJ, Billig H, Tsafriri A. Ovarian follicle atresia: a hormonally controlled apoptotic process. Endocr Rev. 1994;15:707–724. - PubMed
-
- Zeleznik AJ. Follicle selection in primates: “many are called but few are chosen”. Biol Reprod. 2001;65:655–659. - PubMed
-
- Richards JS. Maturation of ovarian follicles: actions and interactions of pituitary and ovarian hormones on follicular cell differentiation. Physiol Rev. 1980;60:51–89. - PubMed
-
- Goodman A, Hodgen G. The ovarian triad of the primate menstrual cycle. Recent Prog Horm Res. 1983;39:1. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

