GM-CSF treated F4/80+ BMCs improve murine hind limb ischemia similar to M-CSF differentiated macrophages
- PMID: 25202910
- PMCID: PMC4159294
- DOI: 10.1371/journal.pone.0106987
GM-CSF treated F4/80+ BMCs improve murine hind limb ischemia similar to M-CSF differentiated macrophages
Abstract
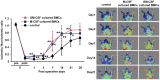
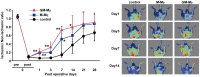
Novel cell therapy is required to treat critical limb ischemia (CLI) as many current approaches require repeated aspiration of bone marrow cells (BMCs). The use of cultured BMCs can reduce the total number of injections required and were shown to induce therapeutic angiogenesis in a murine model of hind limb ischemia. Blood flow recovery was significantly improved in mice treated with granulocyte-macrophage colony-stimulating factor (GM-CSF)-dependent BMCs that secreted inflammatory cytokines. Angiogenesis, lymphangiogenesis, and blood flow recovery ratio were significantly higher in the GM-CSF-cultured F4/80+ macrophage (GM-Mø)-treated group compared with controls. Furthermore, Foxp3+ cell numbers and tissue IL-10 concentrations were significantly increased compared with controls. There was no significant difference in blood flow recovery between GM-Mø and M-CSF-cultured F4/80+ macrophages (M-Mø). Thus, GM-Mø were associated with improved blood flow in hind limb ischemia similar to M-Mø. The selective methods of culturing and treating GM-Mø cells similar to M-Mø cells could be used clinically to help resolve the large number of cells required for BMC treatment of CLI. This study demonstrates a novel cell therapy for CLI that can be used in conjunction with conventional therapy including percutaneous intervention and surgical bypass.
Conflict of interest statement
Figures




Similar articles
-
Granulocyte macrophage-colony stimulating factor: A key modulator of renal mononuclear phagocyte plasticity.Immunobiology. 2019 Jan;224(1):60-74. doi: 10.1016/j.imbio.2018.10.007. Epub 2018 Nov 2. Immunobiology. 2019. PMID: 30415915 Free PMC article.
-
Vascular endothelial growth factor-C derived from CD11b+ cells induces therapeutic improvements in a murine model of hind limb ischemia.J Vasc Surg. 2013 Apr;57(4):1090-9. doi: 10.1016/j.jvs.2012.08.121. Epub 2012 Dec 7. J Vasc Surg. 2013. PMID: 23219511
-
Dependence of interleukin-1-induced arthritis on granulocyte-macrophage colony-stimulating factor.Arthritis Rheum. 2001 Jan;44(1):111-9. doi: 10.1002/1529-0131(200101)44:1<111::AID-ANR15>3.0.CO;2-1. Arthritis Rheum. 2001. PMID: 11212148
-
Proteomic Analysis Reveals Distinct Metabolic Differences Between Granulocyte-Macrophage Colony Stimulating Factor (GM-CSF) and Macrophage Colony Stimulating Factor (M-CSF) Grown Macrophages Derived from Murine Bone Marrow Cells.Mol Cell Proteomics. 2015 Oct;14(10):2722-32. doi: 10.1074/mcp.M115.048744. Epub 2015 Jul 30. Mol Cell Proteomics. 2015. PMID: 26229149 Free PMC article.
-
G-CSF and/or M-CSF accelerate differentiation of bone marrow cells into endothelial progenitor cells in vitro.Oncol Rep. 2006 Jun;15(6):1523-7. Oncol Rep. 2006. PMID: 16685390
Cited by
-
The Role of Macrophages in Vascular Repair and Regeneration after Ischemic Injury.Int J Mol Sci. 2020 Aug 31;21(17):6328. doi: 10.3390/ijms21176328. Int J Mol Sci. 2020. PMID: 32878297 Free PMC article. Review.
-
Recruitment and therapeutic application of macrophages in skeletal muscles after hind limb ischemia.J Vasc Surg. 2018 Jun;67(6):1908-1920.e1. doi: 10.1016/j.jvs.2017.04.070. Epub 2017 Dec 19. J Vasc Surg. 2018. PMID: 29273298 Free PMC article.
-
Myeloid ALX/FPR2 regulates vascularization following tissue injury.Proc Natl Acad Sci U S A. 2020 Jun 23;117(25):14354-14364. doi: 10.1073/pnas.1918163117. Epub 2020 Jun 8. Proc Natl Acad Sci U S A. 2020. PMID: 32513697 Free PMC article.
-
Resolvin D2 Enhances Postischemic Revascularization While Resolving Inflammation.Circulation. 2016 Aug 30;134(9):666-680. doi: 10.1161/CIRCULATIONAHA.116.021894. Epub 2016 Aug 9. Circulation. 2016. PMID: 27507404 Free PMC article.
-
Macrophages: An Inflammatory Link Between Angiogenesis and Lymphangiogenesis.Microcirculation. 2016 Feb;23(2):95-121. doi: 10.1111/micc.12259. Microcirculation. 2016. PMID: 26614117 Free PMC article. Review.
References
-
- Lawall H, Bramlage P, Amann B (2011) Treatment of peripheral arterial disease using stem and progenitor cell therapy. J Vasc Surg 53: 445–453. - PubMed
-
- Annex BH (2013) Therapeutic angiogenesis for critical limb ischaemia. Nat Rev Cardiol 10: 387–396. - PubMed
-
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, et al. (1997) Isolation of putative progenitor endothelial cells for angiogenesis. Science 275: 964–967. - PubMed
-
- Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, et al. (1999) Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res 85: 221–228. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

