Effective removal of staphylococcal biofilms by the endolysin LysH5
- PMID: 25203125
- PMCID: PMC4159335
- DOI: 10.1371/journal.pone.0107307
Effective removal of staphylococcal biofilms by the endolysin LysH5
Abstract
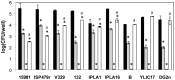
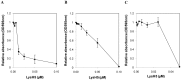
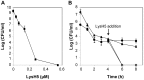
Staphylococcal biofilms are a major concern in both clinical and food settings because they are an important source of contamination. The efficacy of established cleaning procedures is often hindered due to the ability of some antimicrobial compounds to induce biofilm formation, and to the presence of persister cells, a small bacterial subpopulation that exhibits multidrug tolerance. Phage lytic enzymes have demonstrated antimicrobial activity against planktonic and sessile bacteria. However, their ability to lyse and/or select persister cells remains largely unexplored so far. In this work, the lytic activity of the endolysin LysH5 against Staphylococcus aureus and Staphylococcus epidermidis biofilms was confirmed. LysH5 reduced staphylococcal sessile cell counts by 1-3 log units, compared with the untreated control, and sub-inhibitory concentrations of this protein did not induce biofilm formation. LysH5-surviving cells were not resistant to the lytic activity of this protein, suggesting that no persister cells were selected. Moreover, to prove the lytic ability of LysH5 against this subpopulation, both S. aureus exponential cultures and persister cells obtained after treatment with rifampicin and ciprofloxacin were subsequently treated with LysH5. The results demonstrated that besides the notable activity of endolysin LysH5 against staphylococcal biofilms, persister cells were also inhibited, which raises new opportunities as an adjuvant for some antibiotics.
Conflict of interest statement
Figures

References
-
- Lowy FD (1998) Staphylococcus aureus infections. N Engl J Med 339: 520–532. - PubMed
-
- Jabbouri S, Sadovskaya I (2010) Characteristics of the biofilm matrix and its role as a possible target for the detection and eradication of Staphylococcus epidermidis associated with medical implant infections. FEMS Immunol Med Microbiol 59: 280–291. - PubMed
-
- Le Loir Y, Baron F, Gautier M (2003) Staphylococcus aureus and food poisoning. Genet Mol Res 2: 63–76. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

