Automated weaning and SBT systems versus non-automated weaning strategies for weaning time in invasively ventilated critically ill adults
- PMID: 25203308
- PMCID: PMC6516852
- DOI: 10.1002/14651858.CD008638.pub2
Automated weaning and SBT systems versus non-automated weaning strategies for weaning time in invasively ventilated critically ill adults
Abstract
Background: Automated systems use closed-loop control to enable ventilators to perform basic and advanced functions while supporting respiration. SmartCare™ is a unique automated weaning system that measures selected respiratory variables, adapts ventilator output to individual patient needs by operationalizing predetermined algorithms and automatically conducts spontaneous breathing trials (SBTs) when predetermined thresholds are met.
Objectives: The primary objective of this review was to compare weaning time (time from randomization to extubation as defined by study authors) between invasively ventilated critically ill adults weaned by automated weaning and SBT systems versus non-automated weaning strategies.As secondary objectives, we ascertained differences between effects of alternative weaning strategies on clinical outcomes (time to successful extubation, time to first SBT and first successful SBT, mortality, ventilator-associated pneumonia, total duration of ventilation, lengths of intensive care unit (ICU) and hospital stay, use of non-invasive ventilation (NIV), adverse events and clinician acceptance).The third objective of our review was to use subgroup analyses to explore variations in weaning time, length of ICU stay, mortality, ventilator-associated pneumonia, use of NIV and reintubation according to (1) the type of clinician primarily involved in implementing the automated weaning and SBT strategy, (2) the ICU (as a reflection of the population involved) and (3) the non-automated (control) weaning strategy utilized.We conducted a sensitivity analysis to evaluate variations in weaning time based on (4) the methodological quality (low or unclear versus high risk of bias) of the included studies.
Search methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL) 2013, Issue 5; MEDLINE (1966 to 31 May 2013); EMBASE (1988 to 31 May 2013); the Cumulative Index to Nursing and Allied Health Literature (CINAHL) (1982 to 31 May 2013), Evidence-Based Medicine Reviews and Ovid HealthSTAR (1999 to 31 May 2013), as well as conference proceedings and trial registration websites; we also contacted study authors and content experts to identify potentially eligible trials.
Selection criteria: Randomized and quasi-randomized trials comparing automated weaning and SBT systems versus non-automated weaning strategies in intubated adults.
Data collection and analysis: Two review authors independently assessed trial quality and abstracted data according to prespecified criteria. Sensitivity and subgroup analyses were planned to assess the impact on selected outcomes of the following: (1) the type of clinician primarily involved in implementing automated weaning and SBT systems, (2) the ICU (as a reflection of the population involved) and (3) the non-automated (control) weaning strategy utilized.
Main results: We pooled summary estimates from 10 trials evaluating SmartCare™ involving 654 participants. Overall, eight trials were judged to be at low or unclear risk of bias, and two trials were judged to be at high risk of bias. Compared with non-automated strategies, SmartCare™ decreased weaning time (mean difference (MD) -2.68 days, 95% confidence interval (CI) -3.99 to -1.37; P value < 0.0001, seven trials, 495 participants, moderate-quality evidence), time to successful extubation (MD -0.99 days, 95% CI -1.89 to -0.09; P value 0.03, seven trials, 516 participants, low-quality evidence), length of ICU stay (MD -5.70 days, 95% CI -10.54 to -0.85; P value 0.02, six trials, 499 participants, moderate-quality evidence) and proportions of participants receiving ventilation for longer than seven and 21 days (risk ratio (RR) 0.44, 95% CI 0.23 to 0.85; P value 0.01 and RR 0.39, 95% CI 0.18 to 0.86; P value 0.02). SmartCare™ reduced the total duration of ventilation (MD -1.68 days, 95% CI -3.33 to -0.03; P value 0.05, seven trials, 521 participants, low-quality evidence) and the number of participants receiving ventilation for longer than 14 days (RR 0.61, 95% CI 0.37 to 1.00; P value 0.05); however the estimated effects were imprecise. SmartCare™ had no effect on time to first successful SBT, mortality or adverse events, specifically reintubation. Subgroup analysis suggested that trials with protocolized (versus non-protocolized) control weaning strategies reported significantly shorter ICU stays. Sensitivity analysis excluded two trials with high risk of bias and supported a trend toward significant reductions in weaning time favouring SmartCare™.
Authors' conclusions: Compared with non-automated weaning strategies, weaning with SmartCare™ significantly decreased weaning time, time to successful extubation, ICU stay and proportions of patients receiving ventilation for longer than seven days and 21 days. It also showed a favourable trend toward fewer patients receiving ventilation for longer than 14 days; however the estimated effect was imprecise. Summary estimates from our review suggest that these benefits may be achieved without increasing the risk of adverse events, especially reintubation; however, the quality of the evidence ranged from low to moderate, and evidence was derived from 10 small randomized controlled trials.
Conflict of interest statement
Drs Burns and Lellouche hold a $5000 CDN travel bursary from Draeger Medical Inc. (Canada) for the purpose of conducting site visits to participating centres in the WEAN Study. The WEAN Study is an investigator‐initiated trial comparing SmartCare™ and protocolized weaning, for which the co‐principal investigators (Drs Burns and Lellouche) obtained funding from peer‐review, non‐industry sources for implementation. Draeger Medical Inc. provided ventilators and ventilator upgrades for the WEAN study and a central randomization system using electronic mail correspondence (Draeger Medical, Germany). Draeger Medical was not involved in any aspects of study design and oversight, data management or data analysis.
Drs Burns, Lellouche and Lessard have self‐identified as investigators of trials that apply the interventions in question. However, the methods used in conducting this review do not permit bias from these authors in selection, data extraction or risk of bias assessment of any included studies.
Drs Friedrich and Nisenbaum have no conflicts of interest to declare.
Figures
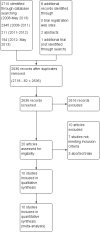
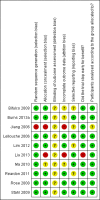



























Update of
- doi: 10.1002/14651858.CD008638
Similar articles
-
Automated weaning and spontaneous breathing trial systems versus non-automated weaning strategies for discontinuation time in invasively ventilated postoperative adults.Cochrane Database Syst Rev. 2014 Feb 13;2014(2):CD008639. doi: 10.1002/14651858.CD008639.pub2. Cochrane Database Syst Rev. 2014. PMID: 24526330 Free PMC article.
-
Automated versus non-automated weaning for reducing the duration of mechanical ventilation for critically ill adults and children.Cochrane Database Syst Rev. 2014 Jun 10;2014(6):CD009235. doi: 10.1002/14651858.CD009235.pub3. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2025 Jul 18;7:CD009235. doi: 10.1002/14651858.CD009235.pub4. PMID: 24915581 Free PMC article. Updated.
-
Automated versus non-automated weaning for reducing the duration of mechanical ventilation for critically ill adults and children.Cochrane Database Syst Rev. 2025 Jul 18;7(7):CD009235. doi: 10.1002/14651858.CD009235.pub4. Cochrane Database Syst Rev. 2025. PMID: 40678933
-
Cough augmentation techniques for extubation or weaning critically ill patients from mechanical ventilation.Cochrane Database Syst Rev. 2017 Jan 11;1(1):CD011833. doi: 10.1002/14651858.CD011833.pub2. Cochrane Database Syst Rev. 2017. PMID: 28075489 Free PMC article.
-
Automated versus non-automated weaning for reducing the duration of mechanical ventilation for critically ill adults and children.Cochrane Database Syst Rev. 2013 Jun 6;(6):CD009235. doi: 10.1002/14651858.CD009235.pub2. Cochrane Database Syst Rev. 2013. Update in: Cochrane Database Syst Rev. 2014 Jun 10;(6):CD009235. doi: 10.1002/14651858.CD009235.pub3. PMID: 23740737 Updated.
Cited by
-
Automated weaning and spontaneous breathing trial systems versus non-automated weaning strategies for discontinuation time in invasively ventilated postoperative adults.Cochrane Database Syst Rev. 2014 Feb 13;2014(2):CD008639. doi: 10.1002/14651858.CD008639.pub2. Cochrane Database Syst Rev. 2014. PMID: 24526330 Free PMC article.
-
Moving from bytes to bedside: a systematic review on the use of artificial intelligence in the intensive care unit.Intensive Care Med. 2021 Jul;47(7):750-760. doi: 10.1007/s00134-021-06446-7. Epub 2021 Jun 5. Intensive Care Med. 2021. PMID: 34089064 Free PMC article.
-
Clinical study replicability and the pursuit of excellence.Crit Care. 2015 Aug 24;19(1):297. doi: 10.1186/s13054-015-1019-1. Crit Care. 2015. PMID: 26299302 Free PMC article.
-
Comparison of ventilatory modes to facilitate liberation from mechanical ventilation: protocol for a systematic review and network meta-analysis.BMJ Open. 2019 Sep 5;9(9):e030407. doi: 10.1136/bmjopen-2019-030407. BMJ Open. 2019. PMID: 31492786 Free PMC article.
-
Identifying Novel Clusters of Patients With Prolonged Mechanical Ventilation Using Trajectories of Rapid Shallow Breathing Index.Front Med (Lausanne). 2022 Jul 4;9:880896. doi: 10.3389/fmed.2022.880896. eCollection 2022. Front Med (Lausanne). 2022. PMID: 35860741 Free PMC article.
References
References to studies included in this review
Bifulco 2008 {published and unpublished data}
-
- Bifulco F, Donato L, Giuricin F, Narni Mancinelli V, Vicchio C, Servillo G. Weaning with SmartCare: our experience. 21st ESICM Annual Congress 2008:S79.
Burns 2013a {published and unpublished data}
-
- Burns KEA, Meade MO, Lessard M, Hand L, Keenan SP, Zhou Q, et al. Wean earlier and automatically with new technology: the WEAN Study. American Journal of Respiratory and Critical Care Medicine. 2013; Vol. 187, issue 11:1203‐11. [PUBMED: 23525929] - PubMed
Jiang 2006 {published and unpublished data}
-
- Jiang H, Yu S, Wang L. Comparison of SmartCare and spontaneous breathing trials for weaning old patients with chronic obstructive pulmonary disease. Chinese Journal of Tuberculosis and Respiratory Diseases 2006;29:545‐8. [PUBMED: 17074269 ] - PubMed
Lellouche 2006 {published and unpublished data}
Lim 2012 {published and unpublished data}
-
- Lim ETS, Hamid N, Beh NC, Phua GC, Tan J. Comparison of knowledge‐based weaning (KBW) and physician‐driven weaning of mechanically ventilated patients in the coronary care unit. European Heart Journal 2012;33:714:P4188.
Liu 2013 {published and unpublished data}
-
- Liu L, Xu X, Yang Y, Huang Y, Liu S, Qiu H. Computer‐driven automated weaning reduces weaning duration in difficult‐to‐wean patients. Chinese Medical Journal 2013;126(10):1814‐8. - PubMed
Ma 2010 {published and unpublished data}
-
- Ma YJ, Yang XJ, Cao XY, Ma XG. Comparison of computer‐driven weaning and physician‐directed weaning from mechanical ventilation: a randomized prospective study. Zhonghua Jie He He Hu Xi Za Zhi 2010;33:174‐8. [PUBMED: 20450634] - PubMed
Reardon 2011 {published and unpublished data}
-
- Reardon C. Clinical Trial of a Computer‐driven Weaning System for Patients Requiring Mechanical Ventilation. Clinical Trials.gov 2011. [Other: NCT00606554]
Rose 2008 {published and unpublished data}
-
- Rose L, Presneill JJ, Johnston L, Cade JF. A randomized, controlled trial of conventional versus automated weaning from mechanical ventilation using SmartCare/PS. Intensive Care Medicine 2008;34:1788‐95. [PUBMED: 18575843 ] - PubMed
Stahl 2009 {published and unpublished data}
-
- Stahl C, Dahmen G, Ziegler A, Muhl E. Comparison of automated protocol‐based versus non‐protocol‐based physician‐directed weaning from mechanical ventilation: a controlled clinical trial. Intensivmed 2009;46:441‐6.
References to studies excluded from this review
Beale 2007 {unpublished data only}
-
- Beale R. Comparison of an automated weaning programme and a standard clinical weaning protocol for weaning critically ill patients. www.controlled‐trials.com 2007. [ISRCTN82559457]
Chen 2008 {published data only}
-
- Chen CW, Wu CP, Dai YL, Perng WC, Huang YC. Adaptive support ventilation (ASV) facilitates ventilator weaning in a medical ICU with limited respiratory therapist support. American Journal of Respiratory and Critical Care Medicine. 2008; Vol. 177:A379.
Donglemans 2007 {published data only}
-
- Dongelmans DA, Veelo DP, Binnekade JM, Vroom MB, Schultz MJ. Pressure controlled/pressure support (PC/PS) versus adaptive support ventilation (ASV) in post‐cardiac surgery patients—a randomized controlled trial. American Journal of Respiratory and Critical Care Medicine. 2007; Vol. 175:A433.
Jolliet 2006 {published data only}
-
- Jolliet P, Battisti A, Roeseler J, Tasseaux D. Automatic adjustment of pressure support (PS) with a computer‐driven knowledge‐based system (SmartCare) during noninvasive ventilation (NIV) in acute respiratory failure. Proceedings of the American Thoracic Society. 2006; Vol. 3:A472.
Jouvet 2007 {published data only}
-
- Jouvet P, Farges C, Hatzakis G, Monir A, Lesage F, Dupic L, Brochard L, et al. Weaning children from mechanical ventilation with a computer‐driven system (closed‐loop protocol): a pilot study. Pediatric Critical Care Medicine 2007;8:425‐32. [PUBMED: 17693913 ] - PubMed
Kataoka 2007 {published data only}
-
- Kataoka G, Murai N, Kodera K, Sasaki A, Asano R, Ikeda M, et al. Clinical experience with SmartCare after off‐pump coronary artery bypass for early extubation. Journal of Artificial Organs 2007;10:218‐22. [PUBMED: 18071851] - PubMed
Papirov 2007 {unpublished data only}
-
- Papirov G. Computer driven management of weaning following prolonged mechanical ventilation: a pilot study. http://clinicaltrials.gov/show/NCT00502489. - PubMed
Schadler 2012 {published data only}
-
- Schadler D, Engel C, Gunnar E, Pulletz S, Haake N, Frerichs I, et al. Automatic control of pressure support for ventilator weaning in surgical intensive care patients. American Journal of Respiratory and Critical Care Medicine 2012;185:637‐44. [PUBMED: 22268137] - PubMed
Taniguchi 2009 {published data only}
Wong 2008 {unpublished data only}
-
- Wong J. Automated Ventilator Controlled Weaning vs Daily Spontaneous Breathing Trial in Difficult to Wean ICU Patients. www.clinicaltrials.gov. December 2008. [NCT00813839]
Additional references
Afessa 1999
-
- Afessa B, Hogans L, Murphy R. Predicting 3‐day and 7‐day outcomes of weaning from mechanical ventilation. Chest 1999;116:456‐61. [PUBMED: 10453876 ] - PubMed
Banner 1997
-
- Banner MJ, Lampotang S, Blanch PB. Mechanical ventilation. In: Civetta JM editor(s). Critical Care. Philadelphia, PA: Lippincott‐Raven, 1997.
Borenstein 2008
-
- Borenstein N, Hedge LV, Higgins JPT, Rothstein HR. Introduction to Meta‐Analysis. Chichester (UK): John Wiley & Sons, 2008.
Bouadma 2005
-
- Bouadma L, Lellouche F, Cabello B, Taille S, Mancebo J, Dojat M, et al. Computer‐driven management of prolonged mechanical ventilation and weaning: a pilot study. Intensive Care Medicine 2005;10:1446‐50. [PUBMED: 16132889] - PubMed
Brochard 1994
-
- Brochard L, Rauss A, Benito S, Conti G, Mancebo J, Rekik N, et al. Comparison of three methods of gradual withdrawal from ventilatory support during weaning from mechanical ventilation. American Journal of Respiratory and Critical Care Medicine 1994;150:896‐903. [MEDLINE: ] - PubMed
Brook 1999
-
- Brook AD, Ahrens TS, Schaiff R, Prentice D, Sherman G, Channon W, et al. Effect of a nursing‐implemented sedation protocol on the duration of mechanical ventilation. Critical Care Medicine 1999;27(12):2609‐15. [PUBMED: 10628598] - PubMed
Burns 2008
-
- Burns KE, Lellouch F, Lessard MR. Automating the weaning process with advanced closed‐loop systems. Intensive Care Medicine 2008;34(10):1757‐65. [MEDLINE: ] - PubMed
Burns 2013b
Butler 1999
-
- Butler R, Keenan SP, Inman KJ, Sibbald WJ, Block G. Is there a preferred technique for weaning the difficult‐to‐wean patient? A systematic review of the literature. Critical Care Medicine 1999;27(11):2331‐6. [PUBMED: 10579244] - PubMed
Chatburn 2004
-
- Chatburn RL. Computer control of mechanical ventilation. Respiratory Care 2004;49(5):507‐17. [MEDLINE: ] - PubMed
Cook 1998
-
- Cook DJ, Walter SD, Cook RJ, Griffith LE, Guyatt GH, Leasa D, et al. Incidence of and risk factors for ventilator‐associated pneumonia in critically ill patients. Annals of Internal Medicine 1998;129(6):433‐40. [MEDLINE: ] - PubMed
Dickersin 1994
Dries 1997
-
- Dries DJ. Weaning from mechanical ventilation. Journal of Trauma, Injury, Infection and Critical Care 1997;43(2):372‐84. [MEDLINE: ] - PubMed
Egger 1997
Ely 1996
-
- Ely EW, Baker AM, Dunagan DP, Burke HL, Smith AC, Kelly PT, et al. Effect of the duration of mechanical ventilation on identifying patients capable of breathing spontaneously. New England Journal of Medicine 1996;335:1864‐9. [MEDLINE: ] - PubMed
Ely 1999
-
- Ely EW, Bennett PA, Bowton DL, Murphy SM, Florance AM, Haponik EF. Large scale implementation of a respiratory therapist‐driven protocol for ventilator weaning. American Journal of Respiratory and Critical Care Medicine 1999;159(2):439‐46. [MEDLINE: ] - PubMed
Epstein 1997
-
- Epstein SK, Ciabotaru RL, Wong JB. Effect of failed extubation on the outcome of mechanical ventilation. Chest 1997;112:186‐92. [MEDLINE: ] - PubMed
Esen 1992
-
- Esen F, Denkel T, Telci L, Kesecioglu J, Tutuncu AS, Akpir K, et al. Comparison of pressure support ventilation and intermittent mandatory ventilation during weaning in patients with acute respiratory failure. Advances in Experimental Medicine and Biology 1992;317:371‐6. [MEDLINE: ] - PubMed
Esteban 1994
-
- Esteban A, Alia I, Ibanez J, Benito S, Tobin MJ. Modes of mechanical ventilation and weaning. A national survey of Spanish hospitals. The Spanish Lung Failure Collaborative Group. Chest 1994;106:1188‐93. [MEDLINE: ] - PubMed
Esteban 1995
-
- Esteban A, Frutos F, Tobin MJ, Alia I, Solsona JF, Valverdu I, et al. A comparison of four methods of weaning patients from mechanical ventilation. Spanish Lung Failure Collaborative Group. New England Journal of Medicine 1995;332:388‐9. [MEDLINE: ] - PubMed
Esteban 1997
-
- Esteban A, Alia I, Gordo F, Fernandez R, Solsona JF, Vallverdu I, et al. Extubation outcome after spontaneous breathing trials with t‐tube or pressure support ventilation. American Journal of Respiratory and Critical Care Medicine 1997;156:459‐65. [MEDLINE: ] - PubMed
Esteban 1999
-
- Esteban A, Alia I, Tobin MJ. Effect of spontaneous breathing trial duration on outcome of attempts to discontinue mechanical ventilation. American Journal of Respiratory and Critical Care Medicine 1999;159:512‐8. [MEDLINE: ] - PubMed
Esteban 2002
-
- Esteban A, Anzueto A, Frutos F, Alia I, Brochard L, Stewart TE, et al. Characteristics and outcomes in adult patients receiving mechanical ventilation: a 28‐d international study. JAMA 2002;287:345‐55. [MEDLINE: ] - PubMed
Guyatt 2008
Heyland 1999
-
- Heyland DK, Cook DJ, Griffith L, Keenan SP, Brun‐Buisson C. The attributable morbidity and mortality of ventilator associated pneumonia in the critically ill patient. The Canadian Critical Care Trials Group. American Journal of Respiratory and Critical Care Medicine 1999;159:1249‐56. [MEDLINE: ] - PubMed
Higgins 2003
Higgins 2011
-
- Higgins JPT, Green S (editors). Chapter 8: Assessing risk of bias in included studies. Section 7.7.3.5 Medians and interquartile ranges. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated September 2008]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Jounieaux 1994
-
- Jounieaux V, Duran A, Levi‐Valensi P. Synchronized intermittent mandatory ventilation with and without pressure support ventilation in weaning patient with COPD from mechanical ventilation. Chest 1994;105:1204‐10. [MEDLINE: ] - PubMed
Kollef 1997
-
- Kollef MH, Shapiro SD, Silver P, John RE, Prentice D, Sauer S, et al. A randomized, controlled trial of protocol‐directed versus physician‐directed weaning from mechanical ventilation. Critical Care Medicine 1997;25:567‐74. [MEDLINE: ] - PubMed
Lefebvre 2001
-
- Lefebvre C, Clarke M. Identifying randomized trials. Egger M, Davey Smith G, Altman D editor(s). Systematic Reviews in Health Care: Meta‐analysis in Context. 2nd Edition. London: BMJ Publishing Group, 2001. [NLM ID 101093083]
Lessard 1996
-
- Lessard MR, Brochard LJ. Weaning from mechanical support. Clinics in Chest Medicine 1996;17(3):475‐89. [MEDLINE: ] - PubMed
Mancebo 1996
-
- Mancebo J. Weaning from mechanical ventilation. European Respiratory Journal 1996;9(9):1923‐31. [MEDLINE: ] - PubMed
Marelich 2000
-
- Marelich GP, Murin S, Battistella F, Inciardi J, Vierra T, Roby M. Protocol weaning of mechanical ventilation in medical and surgical patients by respiratory care practitioners and nurses. Effect on weaning time and incidence of ventilator associated pneumonia. Chest 2000;118:459‐67. [MEDLINE: ] - PubMed
Niederman 1984
-
- Niederman MS, Ferranti RD, Ziegler A, Merrill W, Reynolds HY. Respiratory infection complicating long‐term tracheostomy: the implication of persistent gram‐negative tracheobronchial colonization. Chest 1984;85:39‐44. [MEDLINE: ] - PubMed
Oxman 1992
-
- Oxman A, Guyatt GH. A consumer's guide to subgroup analyses. Annals of Internal Medicine 1992;116(1):78‐84. [MEDLINE: ] - PubMed
Papazian 1996
-
- Papazian L, Bregeon F, Thirion X, Gregoire R, Saux P, Denis JP, et al. Effect of ventilator‐associated pneumonia on mortality and morbidity. American Journal of Respiratory and Critical Care Medicine 1996;154(1):91‐7. [MEDLINE: ] - PubMed
Perren 2002
-
- Perren A, Domenighetti G, Mauri S, Genini F, Vizzardi N. Protocol‐directed weaning from mechanical ventilation; clinical outcome in patients randomized for a 30‐minute and 120‐minute trial with pressure support. Intensive Care Medicine 2002;28:1058‐63. [MEDLINE: ] - PubMed
Pingleton 1988
-
- Pingleton SK. Complications of acute respiratory failure. American Review of Respiratory Diseases 1988;137:1463‐93. [MEDLINE: ] - PubMed
RevMan 5.1 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1.6. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Robinson 2002
-
- Robinson KA, Dickersin K. Development of a highly sensitive search strategy for the retrieval of controlled trials using PubMed. International Journal of Epidemiology 2002;31:150‐3. [MEDLINE: ] - PubMed
Sahn 1973
-
- Sahn SA, Lakshminarayan S. Bedside criteria for discontinuation of mechanical ventilation. Chest 1973;63(6):1002‐5. [MEDLINE: ] - PubMed
Strickland 1991
-
- Strickland JH, Hasson JH. A computer‐controlled ventilator weaning system. Chest 1991;100:1096‐9. [PUBMED: 1914564] - PubMed
Strickland 1993
-
- Strickland JH Jr, Hasson JH. A computer‐controlled ventilator weaning system: a clinical trial. Chest 1993;103:1220‐6. [MEDLINE: ] - PubMed
Stroetz 1995
-
- Stroetz RW, Hubmayer RD. Tidal volume maintenance during weaning with pressure support. American Journal of Respiratory and Critical Care Medicine 1995;152:1034‐40. [MEDLINE: ] - PubMed
Tomlinson 1989
-
- Tomlinson JR, Miller KS, Lorch DF, Smith L, Reines HD, Sahn SA. A prospective comparison of IMV and T‐piece weaning from mechanical ventilation. Chest 1989;96:348‐52. [MEDLINE: ] - PubMed
Vincent 1995
-
- Vincent JL, Bihari DJ, Suter PM, Bruining HA, White J, Nicolas‐Chanoin MH, et al. The prevalence of nosocomial infection in intensive care units in Europe (EPIC). JAMA 1995;274:639‐44. [MEDLINE: ] - PubMed
Vitacca 2000
-
- Vitacca M, Clini E, Porta R, Ambrosino N. Preliminary results on nursing workload in a dedicated weaning center. Intensive Care Medicine 2000;26(6):796‐9. [MEDLINE: ] - PubMed
Yusuf 1991
-
- Yusuf S, Wittes J, Probstfield J, Tyroler HA. Analysis and interpretation of treatment effects in subgroups of patients in randomized clinical trials. Journal of the American Medical Association 1991;266(1):93‐8. [MEDLINE: ] - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

