Survivin expression promotes VEGF-induced tumor angiogenesis via PI3K/Akt enhanced β-catenin/Tcf-Lef dependent transcription
- PMID: 25204429
- PMCID: PMC4177250
- DOI: 10.1186/1476-4598-13-209
Survivin expression promotes VEGF-induced tumor angiogenesis via PI3K/Akt enhanced β-catenin/Tcf-Lef dependent transcription
Abstract
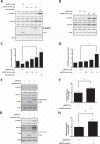
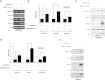
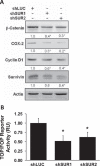
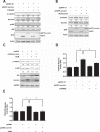
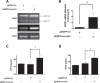
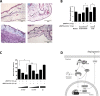
Early in cancer development, tumour cells express vascular endothelial growth factor (VEGF), a secreted molecule that is important in all stages of angiogenesis, an essential process that provides nutrients and oxygen to the nascent tumor and thereby enhances tumor-cell survival and facilitates growth. Survivin, another protein involved in angiogenesis, is strongly expressed in most human cancers, where it promotes tumor survival by reducing apoptosis as well as favoring endothelial cell proliferation and migration. The mechanisms by which cancer cells induce VEGF expression and angiogenesis upon survivin up-regulation remain to be fully established. Since the PI3K/Akt signalling and β-catenin-Tcf/Lef dependent transcription have been implicated in the expression of many cancer-related genes, including survivin and VEGF, we evaluated whether survivin may favor VEGF expression, release from tumor cells and induction of angiogenesis in a PI3K/Akt-β-catenin-Tcf/Lef-dependent manner. Here, we provide evidence linking survivin expression in tumor cells to increased β-catenin protein levels, β-catenin-Tcf/Lef transcriptional activity and expression of several target genes of this pathway, including survivin and VEGF, which accumulates in the culture medium. Alternatively, survivin downregulation reduced β-catenin protein levels and β-catenin-Tcf/Lef transcriptional activity. Also, using inhibitors of PI3K and the expression of dominant negative Akt, we show that survivin acts upstream in an amplification loop to promote VEGF expression. Moreover, survivin knock-down in B16F10 murine melanoma cells diminished the number of blood vessels and reduced VEGF expression in tumors formed in C57BL/6 mice. Finally, in the chick chorioallantoid membrane assay, survivin expression in tumor cells enhanced VEGF liberation and blood vessel formation. Importantly, the presence of neutralizing anti-VEGF antibodies precluded survivin-enhanced angiogenesis in this assay. These findings provide evidence for the existance of a posititve feedback loop connecting survivin expression in tumor cells to PI3K/Akt enhanced β-catenin-Tcf/Lef-dependent transcription followed by secretion of VEGF and angiogenesis.
Figures

Similar articles
-
Caveolin-1-mediated suppression of cyclooxygenase-2 via a beta-catenin-Tcf/Lef-dependent transcriptional mechanism reduced prostaglandin E2 production and survivin expression.Mol Biol Cell. 2009 Apr;20(8):2297-310. doi: 10.1091/mbc.e08-09-0939. Epub 2009 Feb 25. Mol Biol Cell. 2009. PMID: 19244345 Free PMC article.
-
E-cadherin is required for caveolin-1-mediated down-regulation of the inhibitor of apoptosis protein survivin via reduced beta-catenin-Tcf/Lef-dependent transcription.Mol Cell Biol. 2007 Nov;27(21):7703-17. doi: 10.1128/MCB.01991-06. Epub 2007 Sep 4. Mol Cell Biol. 2007. PMID: 17785436 Free PMC article.
-
Glycogen synthase kinase-3beta/beta-catenin promotes angiogenic and anti-apoptotic signaling through the induction of VEGF, Bcl-2 and survivin expression in rat ischemic preconditioned myocardium.J Mol Cell Cardiol. 2006 Jan;40(1):138-47. doi: 10.1016/j.yjmcc.2005.09.009. Epub 2005 Nov 9. J Mol Cell Cardiol. 2006. PMID: 16288908
-
The twisted survivin connection to angiogenesis.Mol Cancer. 2015 Nov 19;14:198. doi: 10.1186/s12943-015-0467-1. Mol Cancer. 2015. PMID: 26584646 Free PMC article. Review.
-
Vasculogenesis and angiogenesis initiation under normoxic conditions through Wnt/β-catenin pathway in gliomas.Rev Neurosci. 2018 Jan 26;29(1):71-91. doi: 10.1515/revneuro-2017-0032. Rev Neurosci. 2018. PMID: 28822229 Review.
Cited by
-
Huaier polysaccharide induces apoptosis in hepatocellular carcinoma cells through p38 MAPK.Oncol Lett. 2016 Aug;12(2):1058-1066. doi: 10.3892/ol.2016.4686. Epub 2016 Jun 8. Oncol Lett. 2016. PMID: 27446394 Free PMC article.
-
Silencing of CARMA3 inhibits bladder cancer cell migration and invasion via deactivating β-catenin signaling pathway.Onco Targets Ther. 2019 Aug 9;12:6309-6322. doi: 10.2147/OTT.S191502. eCollection 2019. Onco Targets Ther. 2019. PMID: 31496734 Free PMC article.
-
Rumen-Protected Glucose Stimulates the Insulin-Like Growth Factor System and mTOR/AKT Pathway in the Endometrium of Early Postpartum Dairy Cows.Animals (Basel). 2020 Feb 23;10(2):357. doi: 10.3390/ani10020357. Animals (Basel). 2020. PMID: 32102173 Free PMC article.
-
Helicobacter pylori-induced inflammation and epigenetic changes during gastric carcinogenesis.World J Gastroenterol. 2015 Dec 7;21(45):12742-56. doi: 10.3748/wjg.v21.i45.12742. World J Gastroenterol. 2015. PMID: 26668499 Free PMC article. Review.
-
Canstatin represses glioma growth by inhibiting formation of VM-like structures.Transl Neurosci. 2021 Aug 10;12(1):309-319. doi: 10.1515/tnsci-2020-0176. eCollection 2021 Jan 1. Transl Neurosci. 2021. PMID: 34434564 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

