PTK7 regulates Id1 expression in CD44-high glioma cells
- PMID: 25204555
- PMCID: PMC4483067
- DOI: 10.1093/neuonc/nou227
PTK7 regulates Id1 expression in CD44-high glioma cells
Abstract
Background: CD44 is a molecular marker associated with molecular subtype and treatment resistance in glioma. More effective therapies will result from approaches aimed at targeting the CD44-high gliomas.
Methods: Protein tyrosine kinase 7 (PTK7) mRNA expression was analyzed based on The Cancer Genome Atlas glioblastoma dataset. PTK7 expression was depleted through lentivirus-mediated short hairpin RNA knockdown. Terminal deoxynucleotidyl transferase dUTP nick-end labeling was used to evaluate cell apoptosis following PTK7 knockdown. Gene expression analysis was performed on Affymetrix microarray. A nude mice orthotopic tumor model was used to evaluate the in vivo effect of PTK7 depletion.
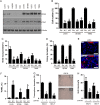
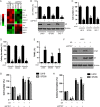
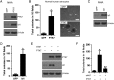
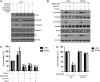
Results: PTK7 is highly expressed in CD44-high glioblastoma and predicts unfavorable prognosis. PTK7 knockdown attenuated cell proliferation, impaired tumorigenic potential, and induced apoptosis in CD44-high glioma cell lines. Gene expression analysis identified inhibitor of DNA Binding 1 (Id1) gene as a potential downstream effector for PTK7. Overexpression of Id1 mostly restored the cell proliferation and colony formation attenuated by PTK7 depletion. PTK7 enhanced anchorage-independent growth in normal human astrocytes, which was attenuated by Id1 knockdown. Furthermore, PTK7 regulated Id1 expression through modulating TGF-β/Smad signaling, while pharmacological inhibition on TGF-β/Smad signaling or PTK7/Id1 depletion attenuated TGF-β-stimulated cell proliferation. PTK7 depletion consistently reduced Id1 expression, suppressed tumor growth, and induced apoptosis in a murine orthotopic tumor model, which could be translated into prolonged survival in tumor-bearing mice.
Conclusions: PTK7 regulates Id1 expression in CD44-high glioma cell lines. Targeting PTK7 could be an effective strategy for treating glioma with high CD44 expression.
Keywords: CD44; PTK7; cell proliferation; glioma; tumorigenesis.
© The Author(s) 2014. Published by Oxford University Press on behalf of the Society for Neuro-Oncology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures


Similar articles
-
TGM2 inhibition attenuates ID1 expression in CD44-high glioma-initiating cells.Neuro Oncol. 2013 Oct;15(10):1353-65. doi: 10.1093/neuonc/not079. Epub 2013 Jul 21. Neuro Oncol. 2013. PMID: 23877317 Free PMC article.
-
Epithelial membrane protein 3 regulates TGF-β signaling activation in CD44-high glioblastoma.Oncotarget. 2017 Feb 28;8(9):14343-14358. doi: 10.18632/oncotarget.11102. Oncotarget. 2017. PMID: 27527869 Free PMC article.
-
TGF-β Receptor Inhibitors Target the CD44(high)/Id1(high) Glioma-Initiating Cell Population in Human Glioblastoma.Cancer Cell. 2010 Dec 14;18(6):655-68. doi: 10.1016/j.ccr.2010.10.023. Cancer Cell. 2010. PMID: 21156287
-
Recent insights into the therapeutic strategies targeting the pseudokinase PTK7 in cancer.Oncogene. 2024 Jun;43(26):1973-1984. doi: 10.1038/s41388-024-03060-x. Epub 2024 May 21. Oncogene. 2024. PMID: 38773263 Free PMC article. Review.
-
G protein-coupled receptors as oncogenic signals in glioma: emerging therapeutic avenues.Neuroscience. 2014 Oct 10;278:222-36. doi: 10.1016/j.neuroscience.2014.08.015. Epub 2014 Aug 24. Neuroscience. 2014. PMID: 25158675 Free PMC article. Review.
Cited by
-
Ligand-modified homologous targeted cancer cell membrane biomimetic nanostructured lipid carriers for glioma therapy.Drug Deliv. 2021 Dec;28(1):2241-2255. doi: 10.1080/10717544.2021.1992038. Drug Deliv. 2021. PMID: 34668811 Free PMC article.
-
Spatio-temporal and Cellular Expression Patterns of PTK7 in the Healthy and Traumatically Injured Rat and Human Spinal Cord.Cell Mol Neurobiol. 2020 Oct;40(7):1087-1103. doi: 10.1007/s10571-020-00794-6. Epub 2020 Jan 23. Cell Mol Neurobiol. 2020. PMID: 31974907 Free PMC article.
-
STAT3-Mediated Promoter-Enhancer Interaction Up-Regulates Inhibitor of DNA Binding 1 (ID1) to Promote Colon Cancer Progression.Int J Mol Sci. 2023 Jun 12;24(12):10041. doi: 10.3390/ijms241210041. Int J Mol Sci. 2023. PMID: 37373188 Free PMC article.
-
The Increased PTK7 Expression Is a Malignant Factor in Cervical Cancer.Dis Markers. 2019 Mar 3;2019:5380197. doi: 10.1155/2019/5380197. eCollection 2019. Dis Markers. 2019. Retraction in: Dis Markers. 2025 May 7;2025:9817180. doi: 10.1155/dim/9817180. PMID: 30944666 Free PMC article. Retracted.
-
Inhibitor of Differentiation 1 (Id1) in Cancer and Cancer Therapy.Int J Med Sci. 2020 Apr 6;17(8):995-1005. doi: 10.7150/ijms.42805. eCollection 2020. Int J Med Sci. 2020. PMID: 32410828 Free PMC article. Review.
References
-
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. - PubMed
-
- Lhoumeau AC, Puppo F, Prébet T, et al. PTK7: a cell polarity receptor with multiple facets. Cell Cycle. 2011;10(8):1233–1236. - PubMed
-
- Mossie K, Jallal B, Alves F, et al. Colon carcinoma kinase-4 defines a new subclass of the receptor tyrosine kinase family. Oncogene. 1995;11(10):2179–2184. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

