Genomic heat shock element sequences drive cooperative human heat shock factor 1 DNA binding and selectivity
- PMID: 25204655
- PMCID: PMC4215228
- DOI: 10.1074/jbc.M114.591578
Genomic heat shock element sequences drive cooperative human heat shock factor 1 DNA binding and selectivity
Abstract
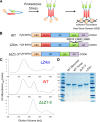
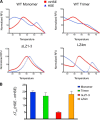
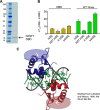
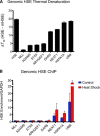
The heat shock transcription factor 1 (HSF1) activates expression of a variety of genes involved in cell survival, including protein chaperones, the protein degradation machinery, anti-apoptotic proteins, and transcription factors. Although HSF1 activation has been linked to amelioration of neurodegenerative disease, cancer cells exhibit a dependence on HSF1 for survival. Indeed, HSF1 drives a program of gene expression in cancer cells that is distinct from that activated in response to proteotoxic stress, and HSF1 DNA binding activity is elevated in cycling cells as compared with arrested cells. Active HSF1 homotrimerizes and binds to a DNA sequence consisting of inverted repeats of the pentameric sequence nGAAn, known as heat shock elements (HSEs). Recent comprehensive ChIP-seq experiments demonstrated that the architecture of HSEs is very diverse in the human genome, with deviations from the consensus sequence in the spacing, orientation, and extent of HSE repeats that could influence HSF1 DNA binding efficacy and the kinetics and magnitude of target gene expression. To understand the mechanisms that dictate binding specificity, HSF1 was purified as either a monomer or trimer and used to evaluate DNA-binding site preferences in vitro using fluorescence polarization and thermal denaturation profiling. These results were compared with quantitative chromatin immunoprecipitation assays in vivo. We demonstrate a role for specific orientations of extended HSE sequences in driving preferential HSF1 DNA binding to target loci in vivo. These studies provide a biochemical basis for understanding differential HSF1 target gene recognition and transcription in neurodegenerative disease and in cancer.
Keywords: Cancer; DNA-binding Protein; Gene Regulation; Neurodegeneration; Transcription Factor.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


References
-
- Bukau B., Weissman J., Horwich A. (2006) Molecular chaperones and protein quality control. Cell 125, 443–451 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

