The membrane attack complex in aging human choriocapillaris: relationship to macular degeneration and choroidal thinning
- PMID: 25204844
- PMCID: PMC4215023
- DOI: 10.1016/j.ajpath.2014.07.017
The membrane attack complex in aging human choriocapillaris: relationship to macular degeneration and choroidal thinning
Abstract
Age-related macular degeneration (AMD) is a common disease that can result in severe visual impairment. Abnormal regulation of the complement system has been implicated in its pathogenesis, and CFH polymorphisms contribute substantially to risk. How these polymorphisms exert their effects is poorly understood. We performed enzyme-linked immunosorbent assay (ELISA) analysis on young, aged, and AMD choroids to determine the abundance of the membrane attack complex (MAC) and performed immunofluorescence studies on eyes from 117 donors to evaluate the MAC in aging, early AMD, and advanced AMD. Morphometric studies were performed on eyes with high- or low-risk CFH genotypes. ELISA confirmed that MAC increases significantly with aging and with AMD. MAC was localized to Bruch's membrane and the choriocapillaris and was detectable at low levels as early as 5 years of age. Hard drusen were labeled with anti-MAC antibody, but large or confluent drusen and basal deposits were generally unlabeled. Labeling of retinal pigment epithelium was observed in some cases of advanced AMD, but not in early disease. Eyes homozygous for the high-risk CFH genotype had thinner choroids than low-risk homozygotes (P < 0.05). These findings suggest that increased complement activation in AMD and in high-risk genotypes can lead to loss of endothelial cells in early AMD. Treatments to protect the choriocapillaris in early AMD are needed.
Figures

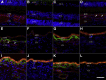
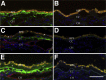
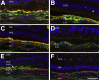

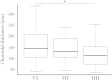
References
-
- Vannewkirk M.R., Nanjan M.B., Wang J.J., Mitchell P., Taylor H.R., McCarty C.A. The prevalence of age-related maculopathy: the Visual Impairment Project. Ophthalmology. 2000;107:1593–1600. - PubMed
-
- Friedman D.S., O’Colmain B.J., Muñoz B., Tomany S.C., McCarty C., de Jong P.T.V.M., Nemesure B., Mitchell P., Kempen J., Eye Diseases Prevalence Research Group Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564–572. [Erratum appeared in Arch Ophthalmol 2011, 129:1188] - PubMed
-
- Bird A.C., Bressler N.M., Bressler S.B., Chisholm I.H., Coscas G., Davis M.D., de Jong P.T.V.M., Klaver C.C.W., Klein B.E.K., Klein R., Mitchell P., Sarks J.P., Sarks S.H., Soubrane G., Taylor H.R., Vingerling J.R., International ARM Epidemiological Study Group An international classification and grading system for age-related maculopathy and age-related macular degeneration. Surv Ophthalmol. 1995;39:367–374. - PubMed
-
- Khandhadia S., Cipriani V., Yates J.R.W., Lotery A.J. Age-related macular degeneration and the complement system. Immunobiology. 2012;217:127–146. - PubMed
-
- Haines J.L., Hauser M.A., Schmidt S., Scott W.K., Olson L.M., Gallins P., Spencer K.L., Kwan S.Y., Noureddine M., Gilbert J.R., Schnetz-Boutaud N., Agarwal A., Postel E.A., Pericak-Vance M.A. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308:419–421. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

