Effect of intensive diabetes therapy on the progression of diabetic retinopathy in patients with type 1 diabetes: 18 years of follow-up in the DCCT/EDIC
- PMID: 25204977
- PMCID: PMC4303965
- DOI: 10.2337/db14-0930
Effect of intensive diabetes therapy on the progression of diabetic retinopathy in patients with type 1 diabetes: 18 years of follow-up in the DCCT/EDIC
Abstract
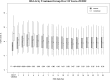
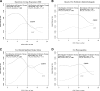
The Diabetes Control and Complications Trial (DCCT) demonstrated that a mean of 6.5 years of intensive therapy aimed at near-normal glucose levels reduced the risk of development and progression of retinopathy by as much as 76% compared with conventional therapy. The Epidemiology of Diabetes Interventions and Complications study (EDIC) observational follow-up showed that the risk of further progression of retinopathy 4 years after the DCCT ended was also greatly reduced in the former intensive group, despite nearly equivalent levels of HbA1c, a phenomenon termed metabolic memory. Metabolic memory was shown to persist through 10 years of follow-up. We now describe the risk of further progression of retinopathy, progression to proliferative diabetic retinopathy, clinically significant macular edema, and the need for intervention (photocoagulation or anti-VEGF) over 18 years of follow-up in EDIC. The cumulative incidence of each retinal outcome continues to be lower in the former intensive group. However, the year-to-year incidence of these outcomes is now similar, owing in large part to a reduction in risk in the former conventional treatment group.
Trial registration: ClinicalTrials.gov NCT00360815 NCT00360893.
© 2015 by the American Diabetes Association. Readers may use this article as long as the work is properly cited, the use is educational and not for profit, and the work is not altered.
Figures

Comment in
-
Further insight on the limits of success of glycemic control in type 1 diabetes.Diabetes. 2015 Feb;64(2):341-3. doi: 10.2337/db14-1447. Diabetes. 2015. PMID: 25614669 Free PMC article. No abstract available.
References
-
- The Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 - PubMed
-
- Diabetes Control and Complications Trial Research Group . Effect of intensive diabetes treatment on the development and progression of long-term complications in adolescents with insulin-dependent diabetes mellitus: Diabetes Control and Complications Trial. J Pediatr 1994;125:177–188 - PubMed
-
- White NH, Cleary PA, Dahms W, Goldstein D, Malone J, Tamborlane WV; Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Research Group . Beneficial effects of intensive therapy of diabetes during adolescence: outcomes after the conclusion of the Diabetes Control and Complications Trial (DCCT). J Pediatr 2001;139:804–812 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

