Role for early-differentiated natural killer cells in infectious mononucleosis
- PMID: 25205117
- PMCID: PMC4199955
- DOI: 10.1182/blood-2014-01-553024
Role for early-differentiated natural killer cells in infectious mononucleosis
Abstract
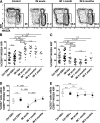
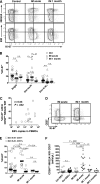
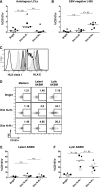
A growing body of evidence suggests that the human natural killer (NK)-cell compartment is phenotypically and functionally heterogeneous and is composed of several differentiation stages. Moreover, NK-cell subsets have been shown to exhibit adaptive immune features during herpes virus infection in experimental mice and to expand preferentially during viral infections in humans. However, both phenotype and role of NK cells during acute symptomatic Epstein-Barr virus (EBV) infection, termed infectious mononucleosis (IM), remain unclear. Here, we longitudinally assessed the kinetics, the differentiation, and the proliferation of subsets of NK cells in pediatric IM patients. Our results indicate that acute IM is characterized by the preferential proliferation of early-differentiated CD56(dim) NKG2A(+) immunoglobulin-like receptor(-) NK cells. Moreover, this NK-cell subset exhibits features of terminal differentiation and persists at higher frequency during at least the first 6 months after acute IM. Finally, we demonstrate that this NK-cell subset preferentially degranulates and proliferates on exposure to EBV-infected B cells expressing lytic antigens. Thus, early-differentiated NK cells might play a key role in the immune control of primary infection with this persistent tumor-associated virus.
© 2014 by The American Society of Hematology.
Figures



References
-
- Björkström NK, Riese P, Heuts F, et al. Expression patterns of NKG2A, KIR, and CD57 define a process of CD56dim NK-cell differentiation uncoupled from NK-cell education. Blood. 2010;116(19):3853–3864. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

