Preclinical development of AMG 139, a human antibody specifically targeting IL-23
- PMID: 25205227
- PMCID: PMC4280975
- DOI: 10.1111/bph.12904
Preclinical development of AMG 139, a human antibody specifically targeting IL-23
Abstract
Background and purpose: AMG 139 is a human anti-IL-23 antibody currently in a phase II trial for treating Crohn's disease. To support its clinical development in humans, in vitro assays and in vivo studies were conducted in cynomolgus monkeys to determine the pharmacology, preclinical characteristics and safety of this monoclonal antibody.
Experimental approach: The in vitro pharmacology, pharmacokinetics (PK), pharmacodynamics and toxicology of AMG 139, after single or weekly i.v. or s.c. administration for up to 26 weeks, were evaluated in cynomolgus monkeys.
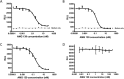
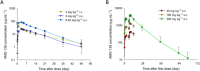
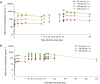
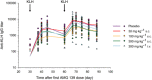
Key results: AMG 139 bound with high affinity to both human and cynomolgus monkey IL-23 and specifically neutralized the biological activity of IL-23 without binding or blocking IL-12. After a single dose, linear PK with s.c. bioavailability of 81% and mean half-life of 8.4-13 days were observed. After weekly s.c. dosing for 3 or 6 months, AMG 139 exposure increased approximately dose-proportionally from 30 to 300 mg·kg(-1) and mean accumulation between the first and last dose ranged from 2- to 3.5-fold. Peripheral blood immunophenotyping, T-cell-dependent antigen responses and bone formation markers were not different between AMG 139 and vehicle treatment. No adverse clinical signs, effects on body weight, vital signs, ophthalmic parameters, clinical pathology, ECG, organ weights or histopathology were observed in the monkeys with the highest dose of AMG 139 tested (300 mg·kg(-1) s.c. or i.v.).
Conclusions and implications: The in vitro pharmacology, PK, immunogenicity and safety characteristics of AMG 139 in cynomolgus monkeys support its continued clinical development for the treatment of various inflammatory diseases.
© 2014 The British Pharmacological Society.
Figures


References
-
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, McGrath JC, et al. The Concise Guide to PHARMACOLOGY 2013/14: Overview. Br J Pharmacol. 2013a;170:1449–1458. - PubMed
-
- Animal and Plant Health Inspection Service USA . In: Animal Welfare Act and Animal Welfare Regulations. Agriculture USDA, editor. Washington, DC: US Department of Agriculture; 2005.
-
- Blumberg H, Dinh H, Dean C, Jr, Trueblood ES, Bailey K, Shows D, et al. IL-1RL2 and its ligands contribute to the cytokine network in psoriasis. J Immunol. 2010;185:4354–4362. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

