Proposed guidelines for the diagnosis and management of methylmalonic and propionic acidemia
- PMID: 25205257
- PMCID: PMC4180313
- DOI: 10.1186/s13023-014-0130-8
Proposed guidelines for the diagnosis and management of methylmalonic and propionic acidemia
Abstract
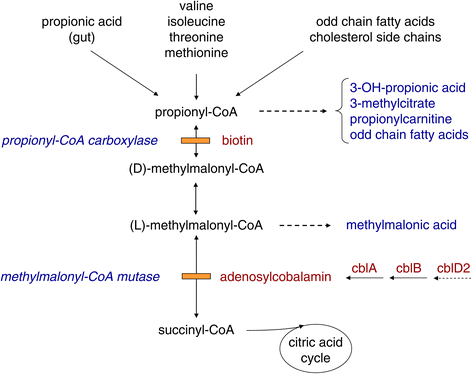
Methylmalonic and propionic acidemia (MMA/PA) are inborn errors of metabolism characterized by accumulation of propionic acid and/or methylmalonic acid due to deficiency of methylmalonyl-CoA mutase (MUT) or propionyl-CoA carboxylase (PCC). MMA has an estimated incidence of ~ 1: 50,000 and PA of ~ 1:100'000 -150,000. Patients present either shortly after birth with acute deterioration, metabolic acidosis and hyperammonemia or later at any age with a more heterogeneous clinical picture, leading to early death or to severe neurological handicap in many survivors. Mental outcome tends to be worse in PA and late complications include chronic kidney disease almost exclusively in MMA and cardiomyopathy mainly in PA. Except for vitamin B12 responsive forms of MMA the outcome remains poor despite the existence of apparently effective therapy with a low protein diet and carnitine. This may be related to under recognition and delayed diagnosis due to nonspecific clinical presentation and insufficient awareness of health care professionals because of disease rarity.
Figures


References
-
- Coelho D, Kim JC, Miousse IR, Fung S, du Moulin M, Buers I, Suormala T, Burda P, Frapolli M, Stucki M, Nürnberg P, Thiele H, Robenek H, Höhne W, Longo N, Pasquali M, Mengel E, Watkins D, Shoubridge EA, Majewski J, Rosenblatt DS, Fowler B, Rutsch F, Baumgartner MR. Mutations in ABCD4 cause a new inborn error of vitamin B12 metabolism. Nat Genet. 2012;44:1152–1155. doi: 10.1038/ng.2386. - DOI - PubMed
-
- Manoli I, Venditti C. Methylmalonic Acidemia. In: Pagon RA, Adam MP, Ardinger HH, Bird TD, Dolan CR, Fong CT, Smith RJH, Stephens K, editors. GeneReviews®. Seattle: University of Washington; 2005.
Publication types
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

