The effect of methylphenidate on neurofibromatosis type 1: a randomised, double-blind, placebo-controlled, crossover trial
- PMID: 25205361
- PMCID: PMC4172829
- DOI: 10.1186/s13023-014-0142-4
The effect of methylphenidate on neurofibromatosis type 1: a randomised, double-blind, placebo-controlled, crossover trial
Abstract
Background: Neurofibromatosis type 1 (NF1) is an autosomal dominant disorder with an estimated prevalence of about 1/3000, independent of ethnicity, race, or gender. Attention Deficit Hyperactivity like Disorder (ADHD)-like characteristics are often reported in patients with NF1. We hypothesised that learning disabilities in NF1 children were related to ADHD symptoms. Treatment with methylphenidate (MPD) has improved learning disabilities in ADHD by acting on neurotransmitters. Our objective was to evaluate its efficacy on ADHD-like symptoms in neurofibromatosis type 1 children (7-12 years).
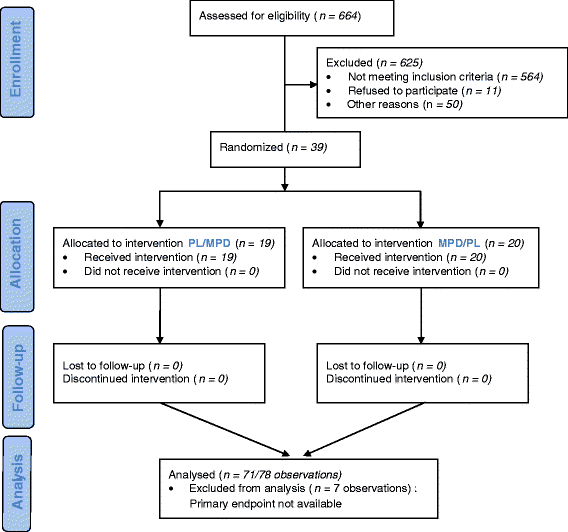
Methods: This was a randomised, double blind, placebo controlled, and crossover trial comparing 0.5 to 0.8 mg/kg/d of MPD as it is indicated for ADHD to placebo in NF1 children with ADHD-like symptoms. Children aged 7 to 12 years were eligible when their IQ was between 80 and 120. The total follow-up was 9 weeks including 4 weeks for each period and 1 week wash out. Fifty subjects (25 for each period) were required for testing the primary study hypothesis. The main outcome was an improvement in scores on the simplified Conners' Parent Rating Scale.
Results: Thirty-nine patients were included between April 2004 and December 2010. Twenty participants received MPD and 19 placebo during the first period. They all completed the trial. MPD decreased the simplified Conners by 3.9 points (±1.1, p = 0. 0003).
Conclusions: This is the first randomised controlled trial showing the short-term benefit of MPD on simplified Conners scores in NF1 children.
Trial registration: ClinicalTrials.gov NCT00169611.
Figures
References
-
- Friedman J, Riccardi VM. Neurofibromatosis: Phenotype, Natural History, and Pathogenesis. 3. Baltimore: The Johns Hopkins University Press; 1999. Clinical and Epidemiological Features; pp. 29–86.
-
- Upadhyaya M, Cooper D. Neurofibromatosis Type 1: Molecular and Cellular Biology. Berlin Heidelberg: Springer; 2012.
-
- North K. Cognitive Function and Academic Performance. In: Friedman JM, Gutmann DH, MacCollin M, Riccardi V, editors. Neurofibromatosis: Phenotype, Natural History, and Pathogenesis. 3. Baltimore: Johns Hopkins University Press; 1999. p. 380.
-
- Pride NA, North KN. The Cognitive Profile of NF1 Children: Therapeutic Implications. In: Cooper MUD, editor. Neurofibromatosis Type 1: Molecular and Cellular Biology. Berlin Heidelberg: Springer; 2012. p. 55.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous