Leucovorin rescue allows effective high-dose pralatrexate treatment and an increase in therapeutic index in mesothelioma xenografts
- PMID: 25205429
- PMCID: PMC4209237
- DOI: 10.1007/s00280-014-2580-z
Leucovorin rescue allows effective high-dose pralatrexate treatment and an increase in therapeutic index in mesothelioma xenografts
Abstract
Purpose: To investigate the ability of leucovorin (LV) to abrogate dose-limiting toxicities of pralatrexate (PDX) while maintaining efficacy, in vivo.
Methods: H2052 mesothelioma cells were treated with the antifolates methotrexate (MTX), PDX and pemetrexed, with and without LV rescue 24 h later. Cell killing was evaluated 48 h later. Female nude mice bearing H2052 xenografts were treated with varying doses and schedules of the antifolate PDX and LV.
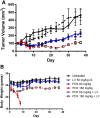
Results: In vitro, H2052 cells were more sensitive to PDX as compared to MTX and pemetrexed. Administration of LV 24 h after antifolate treatment reduced efficacy of antifolates MTX and pemetrexed, but not PDX. In vivo, LV was found to reduce toxicity of PDX at the maximum tolerated dose without sacrificing efficacy. Lethal doses of PDX were rescued by LV, and mice bearing the H2052 tumor demonstrated prolonged and enhanced tumor regression.
Conclusions: High-dose PDX with subsequent LV rescue may be a viable treatment strategy in mesothelioma and other cancers. The inclusion of LV rescue into new and existing PDX treatment protocols should be explored as a way to expand the tolerability and effectiveness of PDX in the clinic.
Figures


Similar articles
-
Distinct mechanistic activity profile of pralatrexate in comparison to other antifolates in in vitro and in vivo models of human cancers.Cancer Chemother Pharmacol. 2009 Oct;64(5):993-9. doi: 10.1007/s00280-009-0954-4. Epub 2009 Feb 17. Cancer Chemother Pharmacol. 2009. PMID: 19221750 Free PMC article.
-
Caffeine markedly sensitizes human mesothelioma cell lines to pemetrexed.Cancer Chemother Pharmacol. 2008 Apr;61(5):819-27. doi: 10.1007/s00280-007-0539-z. Epub 2007 Jun 27. Cancer Chemother Pharmacol. 2008. PMID: 17594092 Free PMC article.
-
A new analogue of 10-deazaaminopterin with markedly enhanced curative effects against human tumor xenografts in mice.Cancer Chemother Pharmacol. 1998;42(4):313-8. doi: 10.1007/s002800050823. Cancer Chemother Pharmacol. 1998. PMID: 9744777
-
[Pemetrexed: from preclinic to clinic].Bull Cancer. 2007;94 Spec No Actualites:S134-8. Bull Cancer. 2007. PMID: 17845983 Review. French.
-
Pemetrexed alone and in combination with platinum compounds in the management of malignant mesothelioma.Clin Lung Cancer. 2004 Apr;5 Suppl 2:S56-60. doi: 10.3816/clc.2004.s.004. Clin Lung Cancer. 2004. PMID: 15117426 Review.
Cited by
-
Histone deacetylase inhibition enhances the therapeutic effects of methotrexate on primary central nervous system lymphoma.Neurooncol Adv. 2020 Jul 3;2(1):vdaa084. doi: 10.1093/noajnl/vdaa084. eCollection 2020 Jan-Dec. Neurooncol Adv. 2020. PMID: 32793886 Free PMC article.
-
Targeting Folate Metabolism Is Selectively Cytotoxic to Glioma Stem Cells and Effectively Cooperates with Differentiation Therapy to Eliminate Tumor-Initiating Cells in Glioma Xenografts.Int J Mol Sci. 2021 Oct 27;22(21):11633. doi: 10.3390/ijms222111633. Int J Mol Sci. 2021. PMID: 34769063 Free PMC article.
-
Generation of pralatrexate resistant T-cell lymphoma lines reveals two patterns of acquired drug resistance that is overcome with epigenetic modifiers.Genes Chromosomes Cancer. 2020 Nov;59(11):639-651. doi: 10.1002/gcc.22884. Epub 2020 Jul 30. Genes Chromosomes Cancer. 2020. PMID: 32614991 Free PMC article.
-
A phase 1 study of romidepsin and pralatrexate reveals marked activity in relapsed and refractory T-cell lymphoma.Blood. 2018 Jan 25;131(4):397-407. doi: 10.1182/blood-2017-09-806737. Epub 2017 Nov 15. Blood. 2018. PMID: 29141948 Free PMC article. Clinical Trial.
-
Methylthioadenosine phosphorylase (MTAP)-deficient T-cell ALL xenografts are sensitive to pralatrexate and 6-thioguanine alone and in combination.Cancer Chemother Pharmacol. 2015 Jun;75(6):1247-52. doi: 10.1007/s00280-015-2747-2. Epub 2015 Apr 28. Cancer Chemother Pharmacol. 2015. PMID: 25917288 Free PMC article.
References
-
- McGuire JJ, Hsieh P, Coward JK, Bertino JR. Enzymatic synthesis of folylpolyglutamates. Characterization of the reaction and its products. J Biol Chem. 1980;255:5776–5788. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

