Novel selective estrogen mimics for the treatment of tamoxifen-resistant breast cancer
- PMID: 25205655
- PMCID: PMC4221411
- DOI: 10.1158/1535-7163.MCT-14-0319
Novel selective estrogen mimics for the treatment of tamoxifen-resistant breast cancer
Abstract
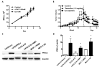
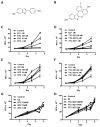
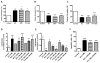
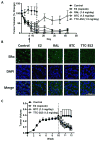
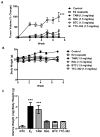
Endocrine-resistant breast cancer is a major clinical obstacle. The use of 17β-estradiol (E2) has reemerged as a potential treatment option following exhaustive use of tamoxifen or aromatase inhibitors, although side effects have hindered its clinical usage. Protein kinase C alpha (PKCα) expression was shown to be a predictor of disease outcome for patients receiving endocrine therapy and may predict a positive response to an estrogenic treatment. Here, we have investigated the use of novel benzothiophene selective estrogen mimics (SEM) as an alternative to E2 for the treatment of tamoxifen-resistant breast cancer. Following in vitro characterization of SEMs, a panel of clinically relevant PKCα-expressing, tamoxifen-resistant models were used to investigate the antitumor effects of these compounds. SEM treatment resulted in growth inhibition and apoptosis of tamoxifen-resistant cell lines in vitro. In vivo SEM treatment induced tumor regression of tamoxifen-resistant T47D:A18/PKCα and T47D:A18-TAM1 tumor models. T47D:A18/PKCα tumor regression was accompanied by translocation of estrogen receptor (ER) α to extranuclear sites, possibly defining a mechanism through which these SEMs initiate tumor regression. SEM treatment did not stimulate growth of E2-dependent T47D:A18/neo tumors. In addition, unlike E2 or tamoxifen, treatment with SEMs did not stimulate uterine weight gain. These findings suggest the further development of SEMs as a feasible therapeutic strategy for the treatment of endocrine-resistant breast cancer without the side effects associated with E2.
©2014 American Association for Cancer Research.
Conflict of interest statement
The authors declare they have no competing interests.
Figures
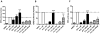
Similar articles
-
Extranuclear ERα is associated with regression of T47D PKCα-overexpressing, tamoxifen-resistant breast cancer.Mol Cancer. 2013 May 1;12:34. doi: 10.1186/1476-4598-12-34. Mol Cancer. 2013. Retraction in: Mol Cancer. 2017 Jul 17;16(1):121. doi: 10.1186/s12943-017-0697-5. PMID: 23634843 Free PMC article. Retracted.
-
Novel antitumor effect of estradiol in athymic mice injected with a T47D breast cancer cell line overexpressing protein kinase Calpha.Clin Cancer Res. 2001 Oct;7(10):3156-65. Clin Cancer Res. 2001. PMID: 11595710
-
Overexpression of PKCalpha is required to impart estradiol inhibition and tamoxifen-resistance in a T47D human breast cancer tumor model.Carcinogenesis. 2006 Aug;27(8):1538-46. doi: 10.1093/carcin/bgl002. Epub 2006 Mar 2. Carcinogenesis. 2006. PMID: 16513679
-
Loss of pigment epithelium-derived factor: a novel mechanism for the development of endocrine resistance in breast cancer.Breast Cancer Res. 2012 Nov 14;14(6):R146. doi: 10.1186/bcr3356. Breast Cancer Res. 2012. Retraction in: Breast Cancer Res. 2023 Sep 19;25(1):107. doi: 10.1186/s13058-023-01711-7. PMID: 23151593 Free PMC article. Retracted.
-
The Divergent Effects of Ovarian Steroid Hormones in the MCF-7 Model for Luminal A Breast Cancer: Mechanistic Leads for Therapy.Int J Mol Sci. 2022 Apr 27;23(9):4800. doi: 10.3390/ijms23094800. Int J Mol Sci. 2022. PMID: 35563193 Free PMC article. Review.
Cited by
-
Revisiting Estrogen for the Treatment of Endocrine-Resistant Breast Cancer: Novel Therapeutic Approaches.Cancers (Basel). 2023 Jul 17;15(14):3647. doi: 10.3390/cancers15143647. Cancers (Basel). 2023. PMID: 37509308 Free PMC article. Review.
-
Estrogen receptor α-NOTCH1 axis enhances basal stem-like cells and epithelial-mesenchymal transition phenotypes in prostate cancer.Cell Commun Signal. 2019 May 23;17(1):50. doi: 10.1186/s12964-019-0367-x. Cell Commun Signal. 2019. PMID: 31122254 Free PMC article.
-
Design and Synthesis of Basic Selective Estrogen Receptor Degraders for Endocrine Therapy Resistant Breast Cancer.J Med Chem. 2019 Dec 26;62(24):11301-11323. doi: 10.1021/acs.jmedchem.9b01580. Epub 2019 Dec 10. J Med Chem. 2019. PMID: 31746603 Free PMC article.
-
Novel Pyrrolopyridone Bromodomain and Extra-Terminal Motif (BET) Inhibitors Effective in Endocrine-Resistant ER+ Breast Cancer with Acquired Resistance to Fulvestrant and Palbociclib.J Med Chem. 2020 Jul 9;63(13):7186-7210. doi: 10.1021/acs.jmedchem.0c00456. Epub 2020 Jun 15. J Med Chem. 2020. PMID: 32453591 Free PMC article.
-
The new biology of estrogen-induced apoptosis applied to treat and prevent breast cancer.Endocr Relat Cancer. 2015 Feb;22(1):R1-31. doi: 10.1530/ERC-14-0448. Epub 2014 Oct 22. Endocr Relat Cancer. 2015. PMID: 25339261 Free PMC article. Review.
References
-
- Dempsey EC, Newton AC, Mochly-Rosen D, Fields AP, Reyland ME, Insel PA, et al. Protein kinase C isozymes and the regulation of diverse cell responses. American Journal of Physiology - Lung Cellular and Molecular Physiology. 2000;279:L429–L38. - PubMed
-
- Mackay HJ, Twelves CJ. Protein kinase C: a target for anticancer drugs? Endocrine-Related Cancer. 2003;10:389–96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

