Promoting endothelial recovery and reducing neointimal hyperplasia using sequential-like release of acetylsalicylic acid and paclitaxel-loaded biodegradable stents
- PMID: 25206303
- PMCID: PMC4157626
- DOI: 10.2147/IJN.S67721
Promoting endothelial recovery and reducing neointimal hyperplasia using sequential-like release of acetylsalicylic acid and paclitaxel-loaded biodegradable stents
Abstract
Introduction: This work reports on the development of a biodegradable dual-drug-eluting stent with sequential-like and sustainable drug-release of anti-platelet acetylsalicylic acid and anti-smooth muscle cell (SMC) proliferative paclitaxel.
Methods: To fabricate the biodegradable stents, poly-L-lactide strips are first cut from a solvent-casted film. They are rolled onto the surface of a metal pin to form spiral stents. The stents are then consecutively covered by acetylsalicylic acid and paclitaxel-loaded polylactide-polyglycolide nanofibers via electrospinning.
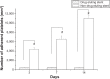
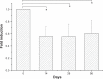
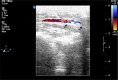
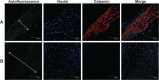
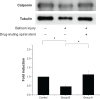
Results: Biodegradable stents exhibit mechanical properties that are superior to those of metallic stents. Biodegradable stents sequentially release high concentrations of acetylsalicylic acid and paclitaxel for more than 30 and 60 days, respectively. In vitro, the eluted drugs promote endothelial cell numbers on days 3 and 7, and reduce the proliferation of SMCs in weeks 2, 4, and 8. The stents markedly inhibit the adhesion of platelets on days 3, 7, and 14 relative to a non-drug-eluting stent. In vivo, the implanted stent is intact, and no stent thrombosis is observed in the stent-implanted vessels without the administration of daily oral acetylsalicylic acid. Promotion of endothelial recovery and inhibition of neointimal hyperplasia are also observed on the stented vessels.
Conclusion: The work demonstrates the efficiency and safety of the biodegradable dual-drug-eluting stents with sequential and sustainable drug release to diseased arteries.
Keywords: biodegradable drug-eluting stents; mechanical properties; poly-L-lactide; polylactide-polyglycolide; sequential-like and sustainable release.
Figures











Similar articles
-
Local sustained delivery of acetylsalicylic acid via hybrid stent with biodegradable nanofibers reduces adhesion of blood cells and promotes reendothelialization of the denuded artery.Int J Nanomedicine. 2014;9:311-26. doi: 10.2147/IJN.S51258. Epub 2014 Jan 6. Int J Nanomedicine. 2014. PMID: 24421640 Free PMC article.
-
Propylthiouracil-coated biodegradable polymer inhibited neointimal formation and enhanced re-endothelialization after vascular injury.Int J Nanomedicine. 2018 Mar 21;13:1761-1771. doi: 10.2147/IJN.S145528. eCollection 2018. Int J Nanomedicine. 2018. PMID: 29606869 Free PMC article.
-
Paclitaxel releasing films consisting of poly(vinyl alcohol)-graft-poly(lactide-co-glycolide) and their potential as biodegradable stent coatings.J Control Release. 2006 Mar 10;111(1-2):235-46. doi: 10.1016/j.jconrel.2005.12.012. Epub 2006 Feb 8. J Control Release. 2006. PMID: 16466824
-
Biodegradable stents as a platform to drug loading.Int J Cardiovasc Intervent. 2003;5(1):13-6. doi: 10.1080/14628840304609. Int J Cardiovasc Intervent. 2003. PMID: 12623560 Review.
-
Drug-eluting stents.Arch Cardiol Mex. 2006 Jul-Sep;76(3):297-319. Arch Cardiol Mex. 2006. PMID: 17091802 Review.
Cited by
-
Computational and experimental mechanical performance of a new everolimus-eluting stent purpose-built for left main interventions.Sci Rep. 2021 Apr 22;11(1):8728. doi: 10.1038/s41598-021-87908-2. Sci Rep. 2021. PMID: 33888765 Free PMC article.
-
Disruptive technological advances in vascular access for dialysis: an overview.Pediatr Nephrol. 2018 Dec;33(12):2221-2226. doi: 10.1007/s00467-017-3853-7. Epub 2017 Nov 29. Pediatr Nephrol. 2018. PMID: 29188361 Review.
-
Vascular Stents Coated with Electrospun Drug-Eluting Material: Functioning in Rabbit Iliac Artery.Polymers (Basel). 2020 Aug 4;12(8):1741. doi: 10.3390/polym12081741. Polymers (Basel). 2020. PMID: 32759856 Free PMC article.
-
Aspirin Inhibits Degenerative Changes of Aneurysmal Wall in a Rat Model.Neurochem Res. 2015 Jul;40(7):1537-45. doi: 10.1007/s11064-015-1603-4. Epub 2015 Jun 21. Neurochem Res. 2015. PMID: 26093650
-
In Vitro Model of Physiological and Pathological Blood Flow with Application to Investigations of Vascular Cell Remodeling.J Vis Exp. 2015 Nov 3;(105):e53224. doi: 10.3791/53224. J Vis Exp. 2015. PMID: 26554396 Free PMC article.
References
-
- Babapulle MN, Eisenberg MJ. Coated stents for the prevention of restenosis: Part I. Circulation. 2002;106(21):2734–2740. - PubMed
-
- Ruygrok PN, Serruys PW. Intracoronary stenting. From concept to custom. Circulation. 1996;94(5):882–890. - PubMed
-
- Hoffmann R, Mintz GS. Coronary in-stent restenosis – predictors, treatment and prevention. Eur Heart J. 2000;21(21):1739–1749. - PubMed
-
- James SK, Stenestrand U, Lindbäck J, et al. SCAAR Study Group Long-term safety and efficacy of drug-eluting versus bare-metal stents in Sweden. N Engl J Med. 2009;360(19):1933–1945. - PubMed
-
- Iakovou I, Schmidt T, Bonizzoni E, et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA. 2005;293(17):2126–2130. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

