Design and production of 3D printed bolus for electron radiation therapy
- PMID: 25207410
- PMCID: PMC5875499
- DOI: 10.1120/jacmp.v15i4.4831
Design and production of 3D printed bolus for electron radiation therapy
Abstract
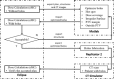
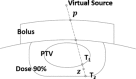
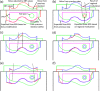
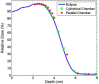
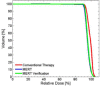
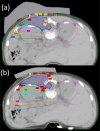
This is a proof-of-concept study demonstrating the capacity for modulated electron radiation therapy (MERT) dose distributions using 3D printed bolus. Previous reports have involved bolus design using an electron pencil beam model and fabrication using a milling machine. In this study, an in-house algorithm is presented that optimizes the dose distribution with regard to dose coverage, conformity, and homogeneity within the planning target volume (PTV). The algorithm takes advantage of a commercial electron Monte Carlo dose calculation and uses the calculated result as input. Distances along ray lines from the distal side of 90% isodose line to distal surface of the PTV are used to estimate the bolus thickness. Inhomogeneities within the calculation volume are accounted for using the coefficient of equivalent thickness method. Several regional modulation operators are applied to improve the dose coverage and uniformity. The process is iterated (usually twice) until an acceptable MERT plan is realized, and the final bolus is printed using solid polylactic acid. The method is evaluated with regular geometric phantoms, anthropomorphic phantoms, and a clinical rhabdomyosarcoma pediatric case. In all cases the dose conformity are improved compared to that with uniform bolus. For geometric phantoms with air or bone inhomogeneities, the dose homogeneity is markedly improved. The actual printed boluses conform well to the surface of complex anthropomorphic phantoms. The correspondence of the dose distribution between the calculated synthetic bolus and the actual manufactured bolus is shown. For the rhabdomyosarcoma patient, the MERT plan yields a reduction of mean dose by 38.2% in left kidney relative to uniform bolus. MERT using 3D printed bolus appears to be a practical, low-cost approach to generating optimized bolus for electron therapy. The method is effective in improving conformity of the prescription isodose surface and in sparing immediately adjacent normal tissues.
Figures











References
-
- Hyödynmaa S, Gustafsson A, Brahme A. Optimization of conformal electron beam therapy using energy‐ and fluence‐modulated beams. Med Phys. 1996;23(5):659–66. - PubMed
-
- Klein EE. Modulated electron beams using multi‐segmented multileaf collimation. Radiother Oncol. 1998;48(3):307–11. - PubMed
-
- Ma CM, Pawlicki T, Lee MC, et al. Energy‐ and intensity‐modulated electron beams for radiotherapy. Phys Med Biol. 2000;45(8):2293–311. - PubMed
-
- Klein EE, Vicic M, Ma CM, Low DA, Drzymala RE. Validation of calculations for electrons modulated with conventional photon multileaf collimators. Phys Med Biol. 2008;53(5):1183–208. - PubMed
-
- Klein EE, Mamalui‐Hunter M, Low DA. Delivery of modulated electron beams with conventional photon multileaf collimators. Phys Med Biol. 2009;54(2):327–39. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

