Molecular physiology of enteric opioid receptors
- PMID: 25207608
- PMCID: PMC4426191
- DOI: 10.1038/ajgsup.2014.5
Molecular physiology of enteric opioid receptors
Abstract
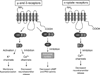
Opioid drugs have powerful antidiarrheal effects and many patients taking these drugs for chronic pain relief experience chronic constipation that can progress to opioid-induced bowel dysfunction. Three classes of opioid receptors are expressed by enteric neurons: μ-, δ-, and κ-opioid receptors (MOR, DOR, and KOR). MOR and DOR couple to inhibition of adenylate cylase and nerve terminal Ca(2+) channels and activation of K(+) channels. These effects reduce neuronal activity and neurotransmitter release. KOR couples to inhibition of Ca(2+) channels and inhibition of neurotransmitter release. In the human gastrointestinal tract, MOR, DOR, and KOR link to inhibition of acetylcholine release from enteric interneurons and purine/nitric oxide release from inhibitory motorneurons. These actions inhibit propulsive motility. MOR and DOR also link to inhibition of submucosal secretomotor neurons, reducing active Cl(-) secretion and passive water movement into the colonic lumen. These effects account for the constipation caused by opioid receptor agonists. Tolerance develops to the analgesic effects of opioid receptor agonists but not to the constipating actions. This may be due to differential β-arrestin-2-dependent opioid receptor desensitization and internalization in enteric nerves in the colon compared with the small intestine and in neuronal pain pathways. Further studies of differential opioid receptor desensitization and tolerance in subsets of enteric neurons may identify new drugs or other treatment strategies of opioid-induced bowel dysfunction.
Conflict of interest statement
Figures

References
-
- Wood JD, Galligan JJ. Function of opioids in the enteric nervous system. Neurogastroenterol Motil. 2004;16(Suppl 2):17–28. - PubMed
-
- Poonyachoti S, Portoghese PS, Brown DR. Characterization of opioid receptors modulating neurogenic contractions of circular muscle from porcine ileum and evidence that delta- and kappa-opioid receptors are coexpressed in myenteric neurons. J Pharmacol Exp Ther. 2001;297:69–77. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
