The binding of apolipoprotein E to oligomers and fibrils of amyloid-β alters the kinetics of amyloid aggregation
- PMID: 25207746
- PMCID: PMC4196732
- DOI: 10.1021/bi5008172
The binding of apolipoprotein E to oligomers and fibrils of amyloid-β alters the kinetics of amyloid aggregation
Abstract
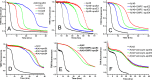
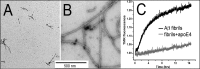
Deposition of amyloid-β (Aβ) in Alzheimer's disease (AD) is strongly correlated with the APOE genotype. However, the role of apolipoprotein E (apoE) in Aβ aggregation has remained unclear. Here we have used different apoE preparations, such as recombinant protein or protein isolated from cultured astrocytes, to examine the effect of apoE on the aggregation of both Aβ1-40 and Aβ1-42. The kinetics of aggregation, measured by the loss of fluorescence of tetramethylrhodamine-labeled Aβ, is shown to be dramatically slowed by the presence of substoichiometric concentrations of apoE. Using these concentrations, we conclude that apoE binds primarily to and affects the growth of oligomers that lead to the nuclei required for fibril growth. At higher apoE concentrations, the protein also binds to Aβ fibrils, resulting in fibril stabilization and a slower rate of fibril growth. The aggregation of Aβ1-40 is dependent on the apoE isoform, being the most dramatic for apoE4 and less so for apoE3 and apoE2. Our results indicate that the detrimental role of apoE4 in AD could be related to apoE-induced stabilization of the soluble but cytotoxic oligomeric forms and intermediates of Aβ, as well as fibril stabilization.
Figures



Similar articles
-
High-affinity multivalent interactions between apolipoprotein E and the oligomers of amyloid-β.FEBS J. 2019 Dec;286(23):4737-4753. doi: 10.1111/febs.14988. Epub 2019 Jul 19. FEBS J. 2019. PMID: 31287614
-
S14G-humanin inhibits Aβ1-42 fibril formation, disaggregates preformed fibrils, and protects against Aβ-induced cytotoxicity in vitro.J Pept Sci. 2013 Mar;19(3):159-65. doi: 10.1002/psc.2484. Epub 2013 Jan 24. J Pept Sci. 2013. Retraction in: J Pept Sci. 2016 Jun;22(6):434. doi: 10.1002/psc.2889. PMID: 23349038 Retracted.
-
Interaction of nascent ApoE2, ApoE3, and ApoE4 isoforms expressed in mammalian cells with amyloid peptide beta (1-40). Relevance to Alzheimer's disease.Biochemistry. 1997 Aug 26;36(34):10571-80. doi: 10.1021/bi9626362. Biochemistry. 1997. PMID: 9265639
-
ApoE and Abeta1-42 interactions: effects of isoform and conformation on structure and function.J Mol Neurosci. 2004;23(3):235-46. doi: 10.1385/JMN:23:3:235. J Mol Neurosci. 2004. PMID: 15181252 Review.
-
The interaction of amyloid-beta with ApoE.Subcell Biochem. 2005;38:255-72. doi: 10.1007/0-387-23226-5_13. Subcell Biochem. 2005. PMID: 15709483 Review.
Cited by
-
rTg-D: A novel transgenic rat model of cerebral amyloid angiopathy Type-2.Cereb Circ Cogn Behav. 2022 Mar 11;3:100133. doi: 10.1016/j.cccb.2022.100133. eCollection 2022. Cereb Circ Cogn Behav. 2022. PMID: 36324401 Free PMC article.
-
ApoE in Alzheimer's disease: pathophysiology and therapeutic strategies.Mol Neurodegener. 2022 Nov 8;17(1):72. doi: 10.1186/s13024-022-00574-4. Mol Neurodegener. 2022. PMID: 36348357 Free PMC article. Review.
-
Morphological analysis of Apolipoprotein E binding to Aβ Amyloid using a combination of Surface Plasmon Resonance, Immunogold Labeling and Scanning Electron Microscopy.BMC Biotechnol. 2019 Dec 11;19(1):97. doi: 10.1186/s12896-019-0589-4. BMC Biotechnol. 2019. PMID: 31829176 Free PMC article.
-
Reduced Influence of apoE on Aβ43 Aggregation and Reduced Vascular Aβ43 Toxicity as Compared with Aβ40 and Aβ42.Mol Neurobiol. 2020 Apr;57(4):2131-2141. doi: 10.1007/s12035-020-01873-x. Epub 2020 Jan 17. Mol Neurobiol. 2020. PMID: 31953617 Free PMC article.
-
ApoE4-specific Misfolded Intermediate Identified by Molecular Dynamics Simulations.PLoS Comput Biol. 2015 Oct 27;11(10):e1004359. doi: 10.1371/journal.pcbi.1004359. eCollection 2015 Oct. PLoS Comput Biol. 2015. PMID: 26506597 Free PMC article.
References
-
- Strittmatter W. J.; Saunders A. M.; Schmechel D.; Pericak-Vance M.; Enghild J.; Salvesen G. S.; Roses A. D. (1993) Apolipoprotein E: High-avidity binding to β-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc. Natl. Acad. Sci. U.S.A. 90, 1977–1981. - PMC - PubMed
-
- Corder E. H.; Saunders A. M.; Pericak-Vance M. A.; Roses A. D. (1995) There is a pathologic relationship between ApoE-ε4 and Alzheimer’s disease. Arch. Neurol. 52, 650–651. - PubMed
-
- Ma J.; Yee A.; Brewer H. B. Jr.; Das S.; Potter H. (1994) Amyloid-associated proteins α1-antichymotrypsin and apolipoprotein E promote assembly of Alzheimer β-protein into filaments. Nature 372, 92–94. - PubMed
-
- Wisniewski T.; Frangione B. (1992) Apolipoprotein E: A pathological chaperone protein in patients with cerebral and systemic amyloid. Neurosci. Lett. 135, 235–238. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

