An isoprenylation and palmitoylation motif promotes intraluminal vesicle delivery of proteins in cells from distant species
- PMID: 25207810
- PMCID: PMC4160200
- DOI: 10.1371/journal.pone.0107190
An isoprenylation and palmitoylation motif promotes intraluminal vesicle delivery of proteins in cells from distant species
Abstract
The C-terminal ends of small GTPases contain hypervariable sequences which may be posttranslationally modified by defined lipid moieties. The diverse structural motifs generated direct proteins towards specific cellular membranes or organelles. However, knowledge on the factors that determine these selective associations is limited. Here we show, using advanced microscopy, that the isoprenylation and palmitoylation motif of human RhoB (-CINCCKVL) targets chimeric proteins to intraluminal vesicles of endolysosomes in human cells, displaying preferential co-localization with components of the late endocytic pathway. Moreover, this distribution is conserved in distant species, including cells from amphibians, insects and fungi. Blocking lipidic modifications results in accumulation of CINCCKVL chimeras in the cytosol, from where they can reach endolysosomes upon release of this block. Remarkably, CINCCKVL constructs are sorted to intraluminal vesicles in a cholesterol-dependent process. In the lower species, neither the C-terminal sequence of RhoB, nor the endosomal distribution of its homologs are conserved; in spite of this, CINCCKVL constructs also reach endolysosomes in Xenopus laevis and insect cells. Strikingly, this behavior is prominent in the filamentous ascomycete fungus Aspergillus nidulans, in which GFP-CINCCKVL is sorted into endosomes and vacuoles in a lipidation-dependent manner and allows monitoring endosomal movement in live fungi. In summary, the isoprenylated and palmitoylated CINCCKVL sequence constitutes a specific structure which delineates an endolysosomal sorting strategy operative in phylogenetically diverse organisms.
Conflict of interest statement
Figures
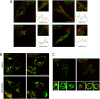

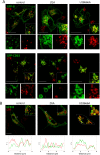


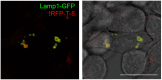
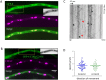
Similar articles
-
Taking a lipidation-dependent path toward endolysosomes.Commun Integr Biol. 2015 Dec 30;8(5):e1078041. doi: 10.1080/19420889.2015.1078041. eCollection 2015 Sep-Oct. Commun Integr Biol. 2015. PMID: 27066167 Free PMC article.
-
Structural determinants allowing endolysosomal sorting and degradation of endosomal GTPases.Traffic. 2010 Sep;11(9):1221-33. doi: 10.1111/j.1600-0854.2010.01091.x. Epub 2010 Jun 21. Traffic. 2010. PMID: 20573066
-
The C-terminal sequence of RhoB directs protein degradation through an endo-lysosomal pathway.PLoS One. 2009 Dec 2;4(12):e8117. doi: 10.1371/journal.pone.0008117. PLoS One. 2009. PMID: 19956591 Free PMC article.
-
Targeting of Specialized Metabolites Biosynthetic Enzymes to Membranes and Vesicles by Posttranslational Palmitoylation: A Mechanism of Non-Conventional Traffic and Secretion of Fungal Metabolites.Int J Mol Sci. 2024 Jan 19;25(2):1224. doi: 10.3390/ijms25021224. Int J Mol Sci. 2024. PMID: 38279221 Free PMC article. Review.
-
Protein sorting into multivesicular endosomes.Curr Opin Cell Biol. 2003 Aug;15(4):446-55. doi: 10.1016/s0955-0674(03)00080-2. Curr Opin Cell Biol. 2003. PMID: 12892785 Review.
Cited by
-
Extracellular vesicle-mediated approaches for the diagnosis and therapy of MASLD: current advances and future prospective.Lipids Health Dis. 2025 Jan 7;24(1):5. doi: 10.1186/s12944-024-02396-3. Lipids Health Dis. 2025. PMID: 39773634 Free PMC article. Review.
-
Taking a lipidation-dependent path toward endolysosomes.Commun Integr Biol. 2015 Dec 30;8(5):e1078041. doi: 10.1080/19420889.2015.1078041. eCollection 2015 Sep-Oct. Commun Integr Biol. 2015. PMID: 27066167 Free PMC article.
-
The role of exosomes contents on genetic and epigenetic alterations of recipient cancer cells.Iran J Basic Med Sci. 2016 Oct;19(10):1031-1039. Iran J Basic Med Sci. 2016. PMID: 27872698 Free PMC article. Review.
-
Post-translational add-ons mark the path in exosomal protein sorting.Cell Mol Life Sci. 2018 Jan;75(1):1-19. doi: 10.1007/s00018-017-2690-y. Epub 2017 Oct 27. Cell Mol Life Sci. 2018. PMID: 29080091 Free PMC article. Review.
-
Endolysosomal impairment by binding of amyloid beta or MAPT/Tau to V-ATPase and rescue via the HYAL-CD44 axis in Alzheimer disease.Autophagy. 2023 Aug;19(8):2318-2337. doi: 10.1080/15548627.2023.2181614. Epub 2023 Feb 26. Autophagy. 2023. PMID: 36843263 Free PMC article.
References
-
- Platta HW, Stenmark H (2011) Endocytosis and signaling. Curr Opin Cell Biol 23: 393–403. - PubMed
-
- Rink J, Ghigo E, Kalaidzidis Y, Zerial M (2005) Rab conversion as a mechanism of progression from early to late endosomes. Cell 122: 735–749. - PubMed
-
- Raiborg C, Stenmark H (2009) The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins. Nature 458: 445–452. - PubMed
-
- Ghosh P, Dahms NM, Kornfeld S (2003) Mannose 6-phosphate receptors: new twists in the tale. Nat Rev Mol Cell Biol 4: 202–212. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

