Percutaneous tumor ablation tools: microwave, radiofrequency, or cryoablation--what should you use and why?
- PMID: 25208284
- PMCID: PMC4319523
- DOI: 10.1148/rg.345140054
Percutaneous tumor ablation tools: microwave, radiofrequency, or cryoablation--what should you use and why?
Abstract
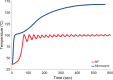
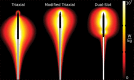
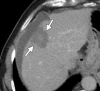
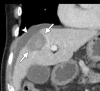
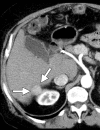
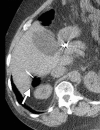
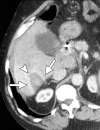
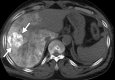
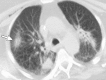
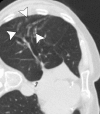
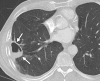
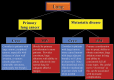
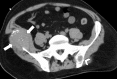
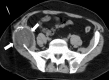
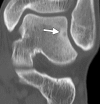
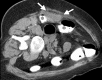
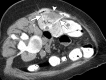
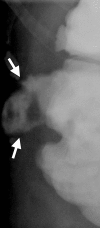
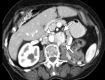
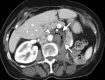
Image-guided thermal ablation is an evolving and growing treatment option for patients with malignant disease of multiple organ systems. Treatment indications have been expanding to include benign tumors as well. Specifically, the most prevalent indications to date have been in the liver (primary and metastatic disease, as well as benign tumors such as hemangiomas and adenomas), kidney (primarily renal cell carcinoma, but also benign tumors such as angiomyolipomas and oncocytomas), lung (primary and metastatic disease), and soft tissue and/or bone (primarily metastatic disease and osteoid osteomas). Each organ system has different underlying tissue characteristics, which can have profound effects on the resulting thermal changes and ablation zone. Understanding these issues is important for optimizing clinical results. In addition, thermal ablation technology has evolved rapidly during the past several decades, with substantial technical and procedural improvements that can help improve clinical outcomes and safety profiles. Staying up to date on these developments is challenging but critical because the physical properties underlying the different ablation modalities and the appropriate use of adjuncts will have a tremendous effect on treatment results. Ultimately, combining an understanding of the physical properties of the ablation modalities with an understanding of the thermal kinetics in tissue and using the most appropriate ablation modality for each patient are key to optimizing clinical outcomes. Suggested algorithms are described that will help physicians choose among the various ablation modalities for individual patients.
©RSNA, 2014.
Figures
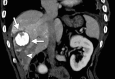

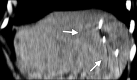
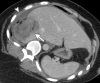
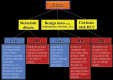

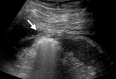
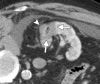
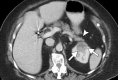
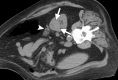
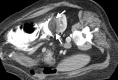
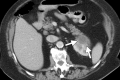
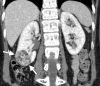
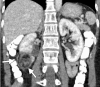
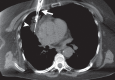
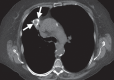
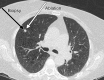
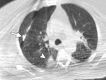


References
-
- Atwell TD, Farrell MA, Callstrom MR, et al. . Percutaneous cryoablation of 40 solid renal tumors with US guidance and CT monitoring: initial experience. Radiology 2007;243(1):276–283. - PubMed
-
- Solbiati L, Ahmed M, Cova L, Ierace T, Brioschi M, Goldberg SN. Small liver colorectal metastases treated with percutaneous radiofrequency ablation: local response rate and long-term survival with up to 10-year follow-up. Radiology 2012;265(3):958–968. - PubMed
-
- Kim SR, Han HJ, Park SJ, et al. . Comparison between surgery and radiofrequency ablation for stage I non-small cell lung cancer. Eur J Radiol 2012;81(2):395–399. - PubMed
-
- Belfiore G, Ronza F, Belfiore MP, et al. . Patients’ survival in lung malignancies treated by microwave ablation: our experience on 56 patients. Eur J Radiol 2013; 82(1):177–181. - PubMed
-
- Woertler K, Vestring T, Boettner F, Winkelmann W, Heindel W, Lindner N. Osteoid osteoma: CT-guided percutaneous radiofrequency ablation and follow-up in 47 patients. J Vasc Interv Radiol 2001;12(6): 717−722. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

