A disease-specific metabolic brain network associated with corticobasal degeneration
- PMID: 25208922
- PMCID: PMC4208467
- DOI: 10.1093/brain/awu256
A disease-specific metabolic brain network associated with corticobasal degeneration
Abstract
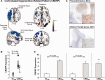
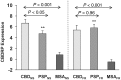
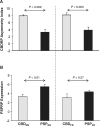
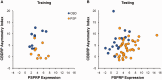
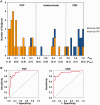
Corticobasal degeneration is an uncommon parkinsonian variant condition that is diagnosed mainly on clinical examination. To facilitate the differential diagnosis of this disorder, we used metabolic brain imaging to characterize a specific network that can be used to discriminate corticobasal degeneration from other atypical parkinsonian syndromes. Ten non-demented patients (eight females/two males; age 73.9 ± 5.7 years) underwent metabolic brain imaging with (18)F-fluorodeoxyglucose positron emission tomography for atypical parkinsonism. These individuals were diagnosed clinically with probable corticobasal degeneration. This diagnosis was confirmed in the three subjects who additionally underwent post-mortem examination. Ten age-matched healthy subjects (five females/five males; age 71.7 ± 6.7 years) served as controls for the imaging studies. Spatial covariance analysis was applied to scan data from the combined group to identify a significant corticobasal degeneration-related metabolic pattern that discriminated (P < 0.001) the patients from the healthy control group. This pattern was characterized by bilateral, asymmetric metabolic reductions involving frontal and parietal cortex, thalamus, and caudate nucleus. These pattern-related changes were greater in magnitude in the cerebral hemisphere opposite the more clinically affected body side. The presence of this corticobasal degeneration-related metabolic topography was confirmed in two independent testing sets of patient and control scans, with elevated pattern expression (P < 0.001) in both disease groups relative to corresponding normal values. We next determined whether prospectively computed expression values for this pattern accurately discriminated corticobasal degeneration from multiple system atrophy and progressive supranuclear palsy (the two most common atypical parkinsonian syndromes) on a single case basis. Based upon this measure, corticobasal degeneration was successfully distinguished from multiple system atrophy (P < 0.001) but not progressive supranuclear palsy, presumably because of the overlap (∼ 24%) that existed between the corticobasal degeneration- and the progressive supranuclear palsy-related metabolic topographies. Nonetheless, excellent discrimination between these disease entities was achieved by computing hemispheric asymmetry scores for the corticobasal degeneration-related pattern on a prospective single scan basis. Indeed, a logistic algorithm based on the asymmetry scores combined with separately computed expression values for a previously validated progressive supranuclear palsy-related pattern provided excellent specificity (corticobasal degeneration: 92.7%; progressive supranuclear palsy: 94.1%) in classifying 58 testing subjects. In conclusion, corticobasal degeneration is associated with a reproducible disease-related metabolic covariance pattern that may help to distinguish this disorder from other atypical parkinsonian syndromes.
Keywords: FDG PET; brain networks; corticobasal degeneration; differential diagnosis; glucose metabolism.
© The Author (2014). Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures
References
-
- Boeve BF. The multiple phenotypes of corticobasal syndrome and corticobasal degeneration: implications for further study. J Mol Neurosci. 2011;45:350–3. - PubMed
-
- Boeve BF, Lang AE, Litvan I. Corticobasal degeneration and its relationship to progressive supranuclear palsy and frontotemporal dementia. Ann Neurol. 2003;54(Suppl 5):S15–9. - PubMed
-
- Boxer AL, Geschwind MD, Belfor N, Gorno-Tempini ML, Schauer GF, Miller BL, et al. Patterns of brain atrophy that differentiate corticobasal degeneration syndrome from progressive supranuclear palsy. Arch Neurol. 2006;63:81–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

