Semaphorin 6B acts as a receptor in post-crossing commissural axon guidance
- PMID: 25209245
- PMCID: PMC6514406
- DOI: 10.1242/dev.112185
Semaphorin 6B acts as a receptor in post-crossing commissural axon guidance
Abstract
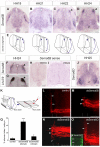
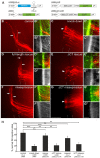
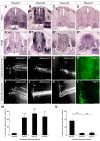
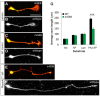
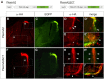
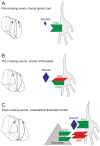
Semaphorins are a large family of axon guidance molecules that are known primarily as ligands for plexins and neuropilins. Although class-6 semaphorins are transmembrane proteins, they have been implicated as ligands in different aspects of neural development, including neural crest cell migration, axon guidance and cerebellar development. However, the specific spatial and temporal expression of semaphorin 6B (Sema6B) in chick commissural neurons suggested a receptor role in axon guidance at the spinal cord midline. Indeed, in the absence of Sema6B, post-crossing commissural axons lacked an instructive signal directing them rostrally along the contralateral floorplate border, resulting in stalling at the exit site or even caudal turns. Truncated Sema6B lacking the intracellular domain was unable to rescue the loss-of-function phenotype, confirming a receptor function of Sema6B. In support of this, we demonstrate that Sema6B binds to floorplate-derived plexin A2 (PlxnA2) for navigation at the midline, whereas a cis-interaction between PlxnA2 and Sema6B on pre-crossing commissural axons may regulate the responsiveness of axons to floorplate-derived cues.
Keywords: Chick; Cis-interaction; In ovo RNAi; PlexinA2; Spinal cord development.
© 2014. Published by The Company of Biologists Ltd.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

