Topography and areal organization of mouse visual cortex
- PMID: 25209296
- PMCID: PMC4160785
- DOI: 10.1523/JNEUROSCI.1124-14.2014
Topography and areal organization of mouse visual cortex
Abstract
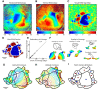
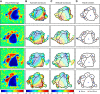
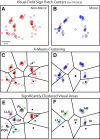
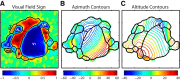
To guide future experiments aimed at understanding the mouse visual system, it is essential that we have a solid handle on the global topography of visual cortical areas. Ideally, the method used to measure cortical topography is objective, robust, and simple enough to guide subsequent targeting of visual areas in each subject. We developed an automated method that uses retinotopic maps of mouse visual cortex obtained with intrinsic signal imaging (Schuett et al., 2002; Kalatsky and Stryker, 2003; Marshel et al., 2011) and applies an algorithm to automatically identify cortical regions that satisfy a set of quantifiable criteria for what constitutes a visual area. This approach facilitated detailed parcellation of mouse visual cortex, delineating nine known areas (primary visual cortex, lateromedial area, anterolateral area, rostrolateral area, anteromedial area, posteromedial area, laterointermediate area, posterior area, and postrhinal area), and revealing two additional areas that have not been previously described as visuotopically mapped in mice (laterolateral anterior area and medial area). Using the topographic maps and defined area boundaries from each animal, we characterized several features of map organization, including variability in area position, area size, visual field coverage, and cortical magnification. We demonstrate that higher areas in mice often have representations that are incomplete or biased toward particular regions of visual space, suggestive of specializations for processing specific types of information about the environment. This work provides a comprehensive description of mouse visuotopic organization and describes essential tools for accurate functional localization of visual areas.
Keywords: extrastriate; imaging; mouse; retinotopy; topography; visual cortex.
Copyright © 2014 the authors 0270-6474/14/3412587-14$15.00/0.
Figures



References
-
- Burwell R, Amaral D. Cortical afferents of the perirhinal, postrhinal, and entorhinal cortices of the rat. J Comp Neurol. 1998;205:179–205. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
