Adeno-associated virus 9-mediated airway expression of antibody protects old and immunodeficient mice against influenza virus
- PMID: 25209558
- PMCID: PMC4248762
- DOI: 10.1128/CVI.00572-14
Adeno-associated virus 9-mediated airway expression of antibody protects old and immunodeficient mice against influenza virus
Abstract
Influenza causes serious and sometimes fatal disease in individuals at risk due to advanced age or immunodeficiencies. Despite progress in the development of seasonal influenza vaccines, vaccine efficacy in elderly and immunocompromised individuals remains low. We recently developed a passive immunization strategy using an adeno-associated virus (AAV) vector to deliver a neutralizing anti-influenza antibody at the site of infection, the nasal airways. Here we show that young, old, and immunodeficient (severe combined immunodeficient [SCID]) mice that were treated intranasally with AAV9 vector expressing a modified version of the broadly neutralizing anti-influenza antibody FI6 were protected and exhibited no signs of disease following an intranasal challenge with the mouse-adapted H1N1 influenza strain A/Puerto Rico/8/1934(H1N1) (PR8) (Mt. Sinai strain). Nonvaccinated mice succumbed to the PR8 challenge due to severe weight loss. We propose that airway-directed AAV9 passive immunization against airborne infectious agents may be beneficial in elderly and immunocompromised patients, for whom there still exists an unmet need for effective vaccination against influenza.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures

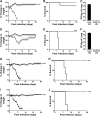
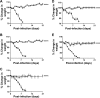
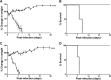
References
-
- Centers for Disease Control and Prevention. 2010. Estimates of deaths associated with seasonal influenza: United States, 1976–2007. MMWR Morb. Mortal. Wkly. Rep. 59(33):1057–1062. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

