Characterization and epitope mapping of the polyclonal antibody repertoire elicited by ricin holotoxin-based vaccination
- PMID: 25209559
- PMCID: PMC4248767
- DOI: 10.1128/CVI.00510-14
Characterization and epitope mapping of the polyclonal antibody repertoire elicited by ricin holotoxin-based vaccination
Abstract
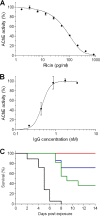
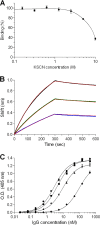
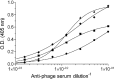
Ricin, one of the most potent and lethal toxins known, is classified by the Centers for Disease Control and Prevention (CDC) as a select agent. Currently, there is no available antidote against ricin exposure, and the most promising therapy is based on neutralizing antibodies elicited by active vaccination or that are given passively. The aim of this study was to characterize the repertoire of anti-ricin antibodies generated in rabbits immunized with ricin toxoid. These anti-ricin antibodies exhibit an exceptionally high avidity (thiocyanate-based avidity index, 9 M) toward ricin and an apparent affinity of 1 nM. Utilizing a novel tissue culture-based assay that enables the determination of ricin activity within a short time period, we found that the anti-ricin antibodies also possess a very high neutralizing titer. In line with these findings, these antibodies conferred mice with full protection against pulmonary ricinosis when administered as a passive vaccination. Epitope mapping analysis using phage display random peptide libraries revealed that the polyclonal serum contains four immunodominant epitopes, three of which are located on the A subunit and one on the B subunit of ricin. Only two of the four epitopes were found to have a significant role in ricin neutralization. To the best of our knowledge, this is the first work that characterizes these immunological aspects of the polyclonal response to ricin holotoxin-based vaccination. These findings provide useful information and a possible strategy for the development and design of an improved ricin holotoxin-based vaccine.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

