Assembly-line synthesis of organic molecules with tailored shapes
- PMID: 25209797
- PMCID: PMC4167605
- DOI: 10.1038/nature13711
Assembly-line synthesis of organic molecules with tailored shapes
Abstract
Molecular 'assembly lines', in which organic molecules undergo iterative processes such as chain elongation and functional group manipulation, are found in many natural systems, including polyketide biosynthesis. Here we report the creation of such an assembly line using the iterative, reagent-controlled homologation of a boronic ester. This process relies on the reactivity of α-lithioethyl tri-isopropylbenzoate, which inserts into carbon-boron bonds with exceptionally high fidelity and stereocontrol; each chain-extension step generates a new boronic ester, which is immediately ready for further homologation. We used this method to generate organic molecules that contain ten contiguous, stereochemically defined methyl groups. Several stereoisomers were synthesized and shown to adopt different shapes-helical or linear-depending on the stereochemistry of the methyl groups. This work should facilitate the rational design of molecules with predictable shapes, which could have an impact in areas of molecular sciences in which bespoke molecules are required.
Figures
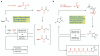

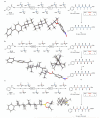


References
-
- Staunton J, Weissman KJ. Polyketide biosynthesis: a millennium review. Nat. Prod. Rep. 2001;18:380–416. - PubMed
-
- Hoffmann RW. Flexible molecules with defined shape - conformational design. Angew. Chem. Int. Ed. Engl. 1992;31:1124–1134.
-
- Hoffmann RW. Conformation design of open-chain compounds. Angew. Chem. Int. Ed. Engl. 2000;39:2054–2070. - PubMed
-
- Smith PW, Still WC. The effect of substitution and stereochemistry on ion binding in the polyether ionophore monensin. J. Am. Chem. Soc. 1988;110:7917–7919.
-
- Wang X, Erickson SD, Iimori T, Still WC. Enantioselective complexation of organic ammonium ions by simple tetracyclic podand ionophores. J. Am. Chem. Soc. 1992;114:4128–4137.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

