New α- and γ-synuclein immunopathological lesions in human brain
- PMID: 25209836
- PMCID: PMC4172890
- DOI: 10.1186/s40478-014-0132-8
New α- and γ-synuclein immunopathological lesions in human brain
Abstract
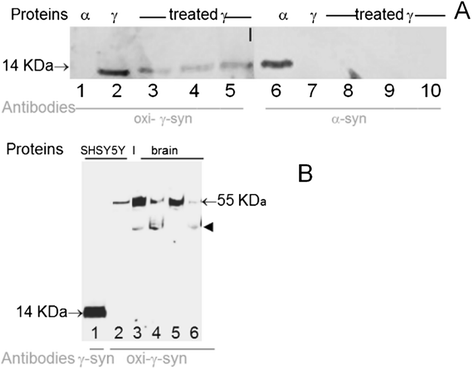
Introduction: Several neurodegenerative diseases are classified as proteopathies as they are associated with the aggregation of misfolded proteins. Synucleinopathies are a group of neurodegenerative disorders associated with abnormal deposition of synucleins. α-Synucleinopathies include Parkinson's disease, dementia with Lewy bodies, and multiple system atrophy. Recently accumulation of another member of the synuclein family- γ-synuclein in neurodegenerative diseases compelled the introduction of the term γ-synucleinopathy. The formation of aggregates and deposits of γ-synuclein is facilitated after its oxidation at methionine 38 (Met38).
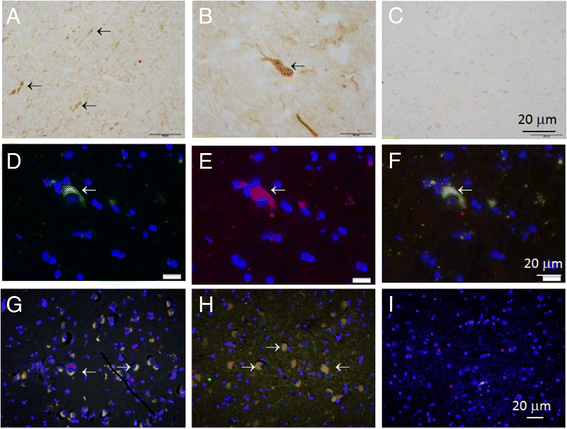
Results: Several types of intracytoplasmic inclusions containing post-translationally modified α- and γ-synucleins are detected. Oxidized Met38-γ-synuclein forms aberrant inclusions in amygdala and substantia nigra. Double staining revealed colocalization of oxidized-γ-synuclein with α-synuclein in the cytoplasm of neurons. Another type of synuclein positive inclusions in the amygdala of dementia with Lewy bodies patients has the appearance of Lewy bodies. These inclusions are immunoreactive when analyzed with antibodies to α-synuclein phosphorylated on serine 129, as well as with antibodies to oxidized-γ-synuclein. Some of these Lewy bodies have doughnut-like shape with round or elongated shape. The separate immunofluorescent images obtained with individual antibodies specific to oxidized-γ-synuclein and phospho-α-synuclein clearly shows the colocalization of these synuclein isoforms in substantia nigra inclusions. Phospho-α-synuclein is present almost exclusively at the periphery of these structures, whereas oxidized-γ-syn immunoreactivity is also located in the internal parts forming dot-like pattern of staining.
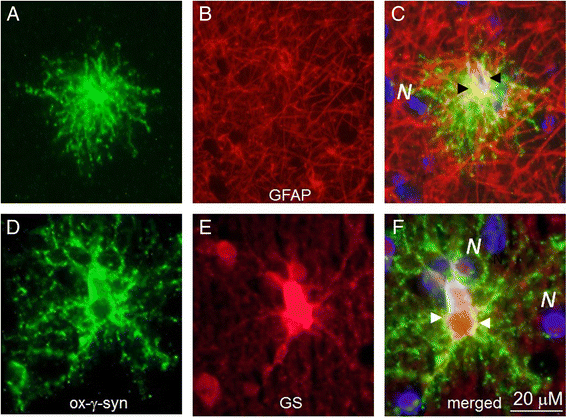
Conclusions: These results reveal new γ-synuclein positive lesions in human brain. Oxidized-γ-synuclein is colocalized with phospho-α-synuclein in doughnut-like inclusions. Several types of astrocytes with different morphology are immunopositive for oxidized-γ-synuclein.
Figures


References
-
- Edison P, Rowe CC, Rinne JO, Ng S, Ahmed I, Kemppainen N, Villemagne VL, O’Keefe G, Någren K, Chaudhury KR, Masters CL, Brooks DJ. Amyloid load in Parkinson’s disease dementia and Lewy body dementia measured with [11C]PIB positron emission tomography. J Neurol Neurosurg Psychiatry. 2008;79(12):1331–1338. doi: 10.1136/jnnp.2007.127878. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

