Implementing the NICE osteoarthritis guidelines: a mixed methods study and cluster randomised trial of a model osteoarthritis consultation in primary care--the Management of OsteoArthritis In Consultations (MOSAICS) study protocol
- PMID: 25209897
- PMCID: PMC4176866
- DOI: 10.1186/s13012-014-0095-y
Implementing the NICE osteoarthritis guidelines: a mixed methods study and cluster randomised trial of a model osteoarthritis consultation in primary care--the Management of OsteoArthritis In Consultations (MOSAICS) study protocol
Abstract
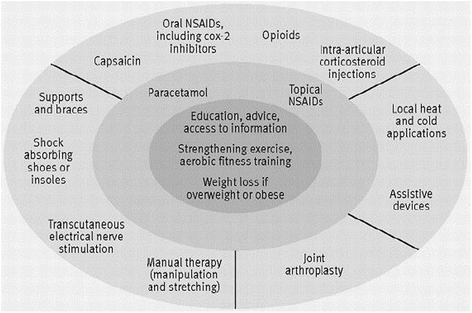
Background: There is as yet no evidence on the feasibility of implementing recommendations from the National Institute of Health and Care Excellence (NICE) osteoarthritis (OA) guidelines in primary care, or of the effect these recommendations have on the condition. The primary aim of this study is to determine the clinical and cost effectiveness of a model OA consultation (MOAC), implementing the core recommendations from the NICE OA guidelines in primary care. Secondary aims are to investigate the impact, feasibility and acceptability of the MOAC intervention; to develop and evaluate a training package for management of OA by general practitioners (GPs) and practice nurses; test the feasibility of deriving 'quality markers' of OA management using a new consultation template and medical record review; and describe the uptake of core NICE OA recommendations in participants aged 45 years and over with joint pain.
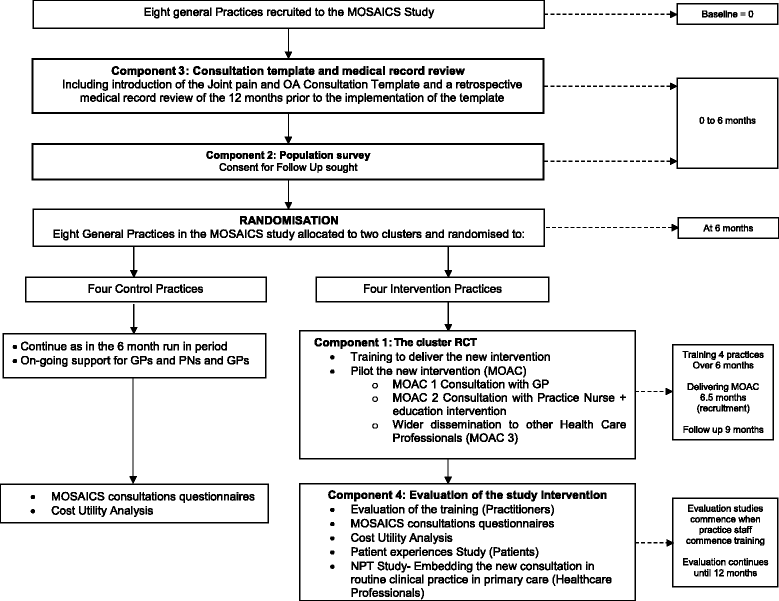
Design: A mixed methods study with a nested cluster randomised controlled trial.
Method: This study was developed according to a defined theoretical framework (the Whole System Informing Self-management Engagement). An overarching model (the Normalisation Process Theory) will be employed to undertake a comprehensive 'whole-system' evaluation of the processes and outcomes of implementing the MOAC intervention. The primary outcome is general physical health (Short Form-12 Physical component score [PCS]) (Ware 1996). The impact, acceptability and feasibility of the MOAC intervention at practice level will be assessed by comparing intervention and control practices using a Quality Indicators template and medical record review. Impact and acceptability of the intervention for patients will be assessed via self-completed outcome measures and semi-structured interviews. The impact, acceptability and feasibility of the MOAC intervention and training for GPs and practice nurses will be evaluated using a variety of methods including questionnaires, semi-structured interviews, and observations.
Discussion: The main output from the study will be to determine whether the MOAC intervention is clinically and cost effective. Additional outputs will be the development of the MOAC for patients consulting with joint pain in primary care, training and educational materials, and resources for patients and professionals regarding supported self-management and uptake of NICE guidance.
Trial registration: ISRCTN number: ISRCTN06984617.
Figures
References
-
- Hill S, Dziedzic K, Thomas E, Baker SR, Croft P. The illness perceptions associated with health and behavioural outcomes in people with musculoskeletal hand problems: findings from the North Staffordshire OA Project (NorStOP) Rheumatology. 2007;46:944–951. doi: 10.1093/rheumatology/kem015. - DOI - PubMed
-
- Jordan KM, Arden NK, Doherty M, Bannwarth B, Bijlsma JWJ, Dieppe P, Gunther K, Hauselmann H, Herrero-Beaumont G, Kaklamanis P, Lohmander S, Leeb B, Lequesne M, Mazieres B, Martin-Mola E, Pavelka K, Pendleton A, Punzi L, Serni U, Swoboda B, Verbruggen G, Zimmerman-Gorska I, Dougados M, Standing Committee for International Clinical Studies Including Therapeutic Trials ESCISIT EULAR Recommendations 2003: an evidence based approach to the management of knee OA: Report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT) Ann Rheum Dis. 2003;62:1145–1155. doi: 10.1136/ard.2003.011742. - DOI - PMC - PubMed
-
- Zhang W, Moskowitz RW, Nuki G, Abramson S, Altman RD, Arden N, Bierma-Zeinstra S, Brandt KD, Croft P, Doherty M, Dougados MD, Hochberg M, Hunter DJ, Kwoh K, Lohmander LS, Tugwell P. OARSI recommendations for the management of hip and knee OA, Part I: Critical appraisal of existing treatment guidelines and systematic review of current research evidence. OA Cartilage. 2007;15:981–1000. doi: 10.1016/j.joca.2007.06.014. - DOI - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

