Survival follow-up and ipilimumab retreatment of patients with advanced melanoma who received ipilimumab in prior phase II studies
- PMID: 25210016
- PMCID: PMC4990834
- DOI: 10.1093/annonc/mdu441
Survival follow-up and ipilimumab retreatment of patients with advanced melanoma who received ipilimumab in prior phase II studies
Abstract
Background: This report provides a survival update at a follow-up of >5 years (5.5-6 years) for patients with advanced melanoma who previously received ipilimumab in phase II clinical trials. Safety and efficacy data following ipilimumab retreatment are also reported.
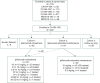
Patients and methods: Patients who previously received ipilimumab 0.3, 3, or 10 mg/kg in one of six phase II trials (CA184-004, CA184-007, CA184-008, CA184-022, MDX010-08, and MDX010-15) were eligible to enroll in the companion study, CA184-025. Upon enrollment, patients initially received ipilimumab retreatment, extended maintenance therapy, or were followed for survival only. Overall survival (OS) rates were evaluated in patients from studies CA184-004, CA184-007, CA184-008, and CA184-022. Safety and best overall response during ipilimumab retreatment at 10 mg/kg were assessed in study CA184-025.
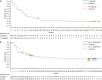
Results: Five-year OS rates for previously treated patients who received ipilimumab induction at 0.3, 3, or 10 mg/kg were 12.3%, 12.3%-16.5%, and 15.5%-28.4%, respectively. Five-year OS rates for treatment-naive patients who received ipilimumab induction at 3 or 10 mg/kg were 26.8% and 21.4%-49.5%, respectively. Little to no change in OS was observed from year 5 up to year 6. The objective response rate among retreated patients was 23%. Grade 3/4 immune-related adverse events occurred in 25%, 5.9%, and 13.2% of retreated patients who initially received ipilimumab 0.3, 3, and 10 mg/kg, with the most common being observed in the skin (4.2%, 2.9%, 3.8%) and gastrointestinal tract (12.5%, 2.9%, 3.8%), respectively.
Conclusions: At a follow-up of 5-6 years, ipilimumab continues to demonstrate durable, long-term survival in a proportion of patients with advanced melanoma. In some patients, ipilimumab retreatment can re-establish disease control with a safety profile that is comparable with that observed during ipilimumab induction. Further studies are needed to determine the contribution of ipilimumab retreatment to OS.
Clinicaltrialsgov: NCT00162123.
Keywords: advanced melanoma; cytotoxic T-lymphocyte antigen-4; immunotherapy; ipilimumab; long-term survival; survival rate.
© The Author 2014. Published by Oxford University Press on behalf of the European Society for Medical Oncology. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures

References
-
- Maio M, Bondarenko I, Robert C, et al. Survival analysis with 5 years of follow-up in a phase III study of ipilimumab and dacarbazine in metastatic melanoma; Amsterdam, The Netherlands: 2013. Presented at the European Cancer Congress 2013, 27 September–1 October 2013 Abstr 3704)
-
- Hoos A, Ibrahim R, Korman A, et al. Development of ipilimumab: contribution to a new paradigm for cancer immunotherapy. Semin Oncol. 2010;37:533–546. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

