Massive thermal acceleration of the emergence of primordial chemistry, the incidence of spontaneous mutation, and the evolution of enzymes
- PMID: 25210030
- PMCID: PMC4215203
- DOI: 10.1074/jbc.R114.567081
Massive thermal acceleration of the emergence of primordial chemistry, the incidence of spontaneous mutation, and the evolution of enzymes
Abstract
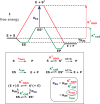
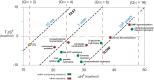
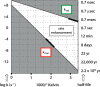
Kelvin considered it unlikely that sufficient time had elapsed on the earth for life to have reached its present level of complexity. In the warm surroundings in which life first appeared, however, elevated temperatures would have reduced the kinetic barriers to reaction. Recent experiments disclose the profound extent to which very slow reactions are accelerated by elevated temperatures, collapsing the time that would have been required for early events in primordial chemistry before the advent of enzymes. If a primitive enzyme, like model catalysts and most modern enzymes, accelerated a reaction by lowering its enthalpy of activation, then the rate enhancement that it produced would have increased automatically as the environment cooled, quite apart from any improvements in catalytic activity that arose from mutation and natural selection. The chemical events responsible for spontaneous mutation are also highly sensitive to temperature, furnishing an independent mechanism for accelerating evolution.
Keywords: Energy of Activation; Enzyme; Enzyme Catalysis; Enzyme Inhibitor; Enzyme Mechanism; Heat of Activation; Spontaneous Mutation; Tempo of Mutation; Thermodynamics.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures

Similar articles
-
Primordial chemistry and enzyme evolution in a hot environment.Cell Mol Life Sci. 2014 Aug;71(15):2909-15. doi: 10.1007/s00018-014-1587-2. Epub 2014 Mar 13. Cell Mol Life Sci. 2014. PMID: 24623557 Free PMC article.
-
Benchmark reaction rates, the stability of biological molecules in water, and the evolution of catalytic power in enzymes.Annu Rev Biochem. 2011;80:645-67. doi: 10.1146/annurev-biochem-060409-093051. Annu Rev Biochem. 2011. PMID: 21495848 Review.
-
Emergence of a Negative Activation Heat Capacity during Evolution of a Designed Enzyme.J Am Chem Soc. 2019 Jul 31;141(30):11745-11748. doi: 10.1021/jacs.9b02731. Epub 2019 Jul 19. J Am Chem Soc. 2019. PMID: 31282667
-
Evolutionary dynamics of enzymes.Protein Eng. 1995 Aug;8(8):791-800. doi: 10.1093/protein/8.8.791. Protein Eng. 1995. PMID: 8637848
-
Hybrid schemes based on quantum mechanics/molecular mechanics simulations goals to success, problems, and perspectives.Adv Protein Chem Struct Biol. 2011;85:81-142. doi: 10.1016/B978-0-12-386485-7.00003-X. Adv Protein Chem Struct Biol. 2011. PMID: 21920322 Review.
Cited by
-
Linear Eyring Plots Conceal a Change in the Rate-Limiting Step in an Enzyme Reaction.Biochemistry. 2018 Dec 11;57(49):6757-6761. doi: 10.1021/acs.biochem.8b01099. Epub 2018 Nov 27. Biochemistry. 2018. PMID: 30472832 Free PMC article.
-
The Classification and Evolution of Enzyme Function.Biophys J. 2015 Sep 15;109(6):1082-6. doi: 10.1016/j.bpj.2015.04.020. Epub 2015 May 15. Biophys J. 2015. PMID: 25986631 Free PMC article. Review.
-
Conformational Sampling of the Intrinsically Disordered C-Terminal Tail of DERA Is Important for Enzyme Catalysis.ACS Catal. 2018 May 4;8(5):3971-3984. doi: 10.1021/acscatal.7b04408. Epub 2018 Mar 27. ACS Catal. 2018. PMID: 30101036 Free PMC article.
-
Identification of Scaffold Specific Energy Transfer Networks in the Enthalpic Activation of Orotidine 5'-Monophosphate Decarboxylase.bioRxiv [Preprint]. 2025 Jan 29:2025.01.29.635545. doi: 10.1101/2025.01.29.635545. bioRxiv. 2025. PMID: 39975186 Free PMC article. Preprint.
-
Rapid bursts and slow declines: on the possible evolutionary trajectories of enzymes.J R Soc Interface. 2015 Jun 6;12(107):20150036. doi: 10.1098/rsif.2015.0036. J R Soc Interface. 2015. PMID: 25926697 Free PMC article. Review.
References
-
- Wolfenden R. (1972) Analog approaches to the transition state in enzyme reactions. Acc. Chem. Res. 5, 10–18
-
- Wolfenden R. (2003) Thermodynamic and extrathermodynamic requirements of enzyme catalysis. Biophys. Chem. 105, 559–572 - PubMed
-
- Kahne D., Still W. C. (1988) Hydrolysis of a peptide bond in neutral water. J. Am. Chem. Soc. 110, 7529–7534
-
- Harcourt A. V., Esson W. T. (1866) On the laws of connexion between the conditions of a chemical change and its amount. Phil. Trans. R. Soc. London 156, 193–221
-
- Radzicka A., Wolfenden R., (1995) A proficient enzyme. Science 267, 90–93 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

