Human 2-oxoglutarate dehydrogenase complex E1 component forms a thiamin-derived radical by aerobic oxidation of the enamine intermediate
- PMID: 25210035
- PMCID: PMC4207997
- DOI: 10.1074/jbc.M114.591073
Human 2-oxoglutarate dehydrogenase complex E1 component forms a thiamin-derived radical by aerobic oxidation of the enamine intermediate
Abstract
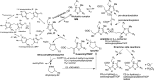
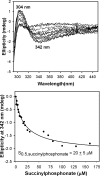
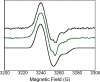
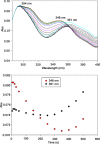
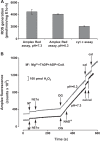
Herein are reported unique properties of the human 2-oxoglutarate dehydrogenase multienzyme complex (OGDHc), a rate-limiting enzyme in the Krebs (citric acid) cycle. (a) Functionally competent 2-oxoglutarate dehydrogenase (E1o-h) and dihydrolipoyl succinyltransferase components have been expressed according to kinetic and spectroscopic evidence. (b) A stable free radical, consistent with the C2-(C2α-hydroxy)-γ-carboxypropylidene thiamin diphosphate (ThDP) cation radical was detected by electron spin resonance upon reaction of the E1o-h with 2-oxoglutarate (OG) by itself or when assembled from individual components into OGDHc. (c) An unusual stability of the E1o-h-bound C2-(2α-hydroxy)-γ-carboxypropylidene thiamin diphosphate (the "ThDP-enamine"/C2α-carbanion, the first postdecarboxylation intermediate) was observed, probably stabilized by the 5-carboxyl group of OG, not reported before. (d) The reaction of OG with the E1o-h gave rise to superoxide anion and hydrogen peroxide (reactive oxygen species (ROS)). (e) The relatively stable enzyme-bound enamine is the likely substrate for oxidation by O2, leading to the superoxide anion radical (in d) and the radical (in b). (f) The specific activity assessed for ROS formation compared with the NADH (overall complex) activity, as well as the fraction of radical intermediate occupying active centers of E1o-h are consistent with each other and indicate that radical/ROS formation is an "off-pathway" side reaction comprising less than 1% of the "on-pathway" reactivity. However, the nearly ubiquitous presence of OGDHc in human tissues, including the brain, makes these findings of considerable importance in human metabolism and perhaps disease.
Keywords: Dehydrogenase; Electron Paramagnetic Resonance (EPR); Mass Spectrometry (MS); Reactive Oxygen Species (ROS); Thiamin.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures







References
-
- Perham R. N. (2000) Swinging arms and swinging domains in multifunctional enzymes: catalytic machines for multistep reactions. Annu. Rev. Biochem. 69, 961–1004 - PubMed
-
- Reed L. J. (2001) A trial of research from lipoic acid to α-keto acid dehydrogenase complexes. J. Biol. Chem. 276, 38329–38336 - PubMed
-
- Perham R. N. (1991) Domains, motifs, and linkers in 2-oxo acid dehydrogenase multienzyme complexes: a paradigm in the design of a multifunctional protein. Biochemistry 30, 8501–8512 - PubMed
-
- Gibson G. E., Park L. C. H., Sheu K. F. R., Blass J. P., Calingasan N. Y. (2000) The α-ketoglutarate dehydrogenase complex in neurodegeneration. Neurochem. Int. 36, 97–112 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

