Mechanisms of neutralization of a human anti-α-toxin antibody
- PMID: 25210036
- PMCID: PMC4207998
- DOI: 10.1074/jbc.M114.601328
Mechanisms of neutralization of a human anti-α-toxin antibody
Abstract
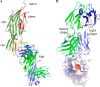
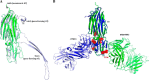
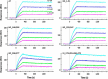
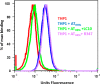
MEDI4893 is a neutralizing human monoclonal antibody that targets α-toxin (AT) and is currently undergoing evaluation in the field of Staphylococcus aureus-mediated diseases. We have solved the crystal structure of MEDI4893 Fab bound to monomeric AT at a resolution of 2.56 Å and further characterized its epitope using various engineered AT variants. We have found that MEDI4893 recognizes a novel epitope in the so-called "rim" domain of AT and exerts its neutralizing effect through a dual mechanism. In particular, MEDI4893 not only sterically blocks binding of AT to its cell receptor but also prevents it from adopting a lytic heptameric trans-membrane conformation.
Keywords: Alpha Toxin; Antibody; Crystal Structure; Fab; Infectious Disease; Mutagenesis; Staphylococcus aureus (S. aureus).
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures
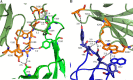


References
-
- Lowy F. D. (1998) Staphylococcus aureus infections. N. Engl. J. Med. 339, 520–532 - PubMed
-
- Klevens R. M., Morrison M. A., Nadle J., Petit S., Gershman K., Ray S., Harrison L. H., Lynfield R., Dumyati G., Townes J. M., Craig A. S., Zell E. R., Fosheim G. E., McDougal L. K., Carey R. B., Fridkin S. K. (2007) Invasive methicillin-resistant Staphylococcus aureus infections in the United States. Active Bacterial Core surveillance (ABCs) MRSA Investigators. JAMA 298, 1763–1771 - PubMed
-
- Fowler V. G., Jr., Olsen M. K., Corey G. R., Woods C. W., Cabell C. H., Reller L. B., Cheng A. C., Dudley T., Oddone E. Z. (2003) Clinical identifiers of complicated Staphylococcus aureus bacteremia. Arch. Intern. Med. 163, 2066–2072 - PubMed
-
- de Kraker M. E., Wolkewitz M., Davey P. G., Koller W., Berger J., Nagler J., Icket C., Kalenic S., Horvatic J., Seifert H., Kaasch A. J., Paniara O., Argyropoulou A., Bompola M., Smyth E., Skally M., Raglio A., Dumpis U., Kelmere A. M., Borg M., Xuereb D., Ghita M. C., Noble M., Kolman J., Grabljevec S., Turner D., Lansbury L., Grundmann H. (2011) Clinical impact of antimicrobial resistance in European hospitals: excess mortality and length of hospital stay related to methicillin-resistant Staphylococcus aureus bloodstream infections. Antimicrob. Agents Chemother. 55, 1598–1605 - PMC - PubMed
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

