Endoplasmic reticulum-associated inactivation of the hormone jasmonoyl-L-isoleucine by multiple members of the cytochrome P450 94 family in Arabidopsis
- PMID: 25210037
- PMCID: PMC4207986
- DOI: 10.1074/jbc.M114.603084
Endoplasmic reticulum-associated inactivation of the hormone jasmonoyl-L-isoleucine by multiple members of the cytochrome P450 94 family in Arabidopsis
Abstract
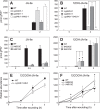
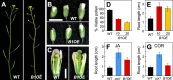
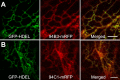
The plant hormone jasmonate (JA) controls diverse aspects of plant immunity, growth, and development. The amplitude and duration of JA responses are controlled in large part by the intracellular level of jasmonoyl-L-isoleucine (JA-Ile). In contrast to detailed knowledge of the JA-Ile biosynthetic pathway, little is known about enzymes involved in JA-Ile metabolism and turnover. Cytochromes P450 (CYP) 94B3 and 94C1 were recently shown to sequentially oxidize JA-Ile to hydroxy (12OH-JA-Ile) and dicarboxy (12COOH-JA-Ile) derivatives. Here, we report that a third member (CYP94B1) of the CYP94 family also participates in oxidative turnover of JA-Ile in Arabidopsis. In vitro studies showed that recombinant CYP94B1 converts JA-Ile to 12OH-JA-Ile and lesser amounts of 12COOH-JA-Ile. Consistent with this finding, metabolic and physiological characterization of CYP94B1 loss-of-function and overexpressing plants demonstrated that CYP94B1 and CYP94B3 coordinately govern the majority (>95%) of 12-hydroxylation of JA-Ile in wounded leaves. Analysis of CYP94-promoter-GUS reporter lines indicated that CYP94B1 and CYP94B3 serve unique and overlapping spatio-temporal roles in JA-Ile homeostasis. Subcellular localization studies showed that CYP94s involved in conversion of JA-Ile to 12COOH-JA-Ile reside on endoplasmic reticulum (ER). In vitro studies further showed that 12COOH-JA-Ile, unlike JA-Ile, fails to promote assembly of COI1-JAZ co-receptor complexes. The double loss-of-function mutant of CYP94B3 and ILL6, a JA-Ile amidohydrolase, displayed a JA profile consistent with the collaborative action of the oxidative and the hydrolytic pathways in JA-Ile turnover. Collectively, our results provide an integrated view of how multiple ER-localized CYP94 and JA amidohydrolase enzymes attenuate JA signaling during stress responses.
Keywords: Cytochrome P450; Fatty Acid Oxidation; Jasmonate; Plant Biochemistry; Plant Hormone; Stress Response.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




Similar articles
-
Cytochromes P450 CYP94C1 and CYP94B3 catalyze two successive oxidation steps of plant hormone Jasmonoyl-isoleucine for catabolic turnover.J Biol Chem. 2012 Feb 24;287(9):6296-306. doi: 10.1074/jbc.M111.316364. Epub 2012 Jan 3. J Biol Chem. 2012. PMID: 22215670 Free PMC article.
-
Sequential oxidation of Jasmonoyl-Phenylalanine and Jasmonoyl-Isoleucine by multiple cytochrome P450 of the CYP94 family through newly identified aldehyde intermediates.Phytochemistry. 2015 Sep;117:388-399. doi: 10.1016/j.phytochem.2015.06.027. Epub 2015 Jul 9. Phytochemistry. 2015. PMID: 26164240
-
CYP94-mediated jasmonoyl-isoleucine hormone oxidation shapes jasmonate profiles and attenuates defence responses to Botrytis cinerea infection.J Exp Bot. 2015 Jul;66(13):3879-92. doi: 10.1093/jxb/erv190. Epub 2015 Apr 22. J Exp Bot. 2015. PMID: 25903915 Free PMC article.
-
Chemical and genetic exploration of jasmonate biosynthesis and signaling paths.Planta. 2012 Nov;236(5):1351-66. doi: 10.1007/s00425-012-1705-z. Epub 2012 Jul 28. Planta. 2012. PMID: 23011567 Review.
-
Jasmonate passes muster: a receptor and targets for the defense hormone.Annu Rev Plant Biol. 2009;60:183-205. doi: 10.1146/annurev.arplant.043008.092007. Annu Rev Plant Biol. 2009. PMID: 19025383 Review.
Cited by
-
Novel insights into maize (Zea mays) development and organogenesis for agricultural optimization.Planta. 2023 Apr 9;257(5):94. doi: 10.1007/s00425-023-04126-y. Planta. 2023. PMID: 37031436 Review.
-
Arabidopsis JASMONATE-INDUCED OXYGENASES down-regulate plant immunity by hydroxylation and inactivation of the hormone jasmonic acid.Proc Natl Acad Sci U S A. 2017 Jun 13;114(24):6388-6393. doi: 10.1073/pnas.1701101114. Epub 2017 May 30. Proc Natl Acad Sci U S A. 2017. PMID: 28559313 Free PMC article.
-
Multi-tissue transcriptome analysis using hybrid-sequencing reveals potential genes and biological pathways associated with azadirachtin A biosynthesis in neem (azadirachta indica).BMC Genomics. 2020 Oct 28;21(1):749. doi: 10.1186/s12864-020-07124-6. BMC Genomics. 2020. PMID: 33115410 Free PMC article.
-
Salicylic acid and jasmonic acid in plant immunity.Hortic Res. 2025 Mar 11;12(7):uhaf082. doi: 10.1093/hr/uhaf082. eCollection 2025 Jul. Hortic Res. 2025. PMID: 40343347 Free PMC article.
-
Jasmonic Acid Signaling and Molecular Crosstalk with Other Phytohormones.Int J Mol Sci. 2021 Mar 13;22(6):2914. doi: 10.3390/ijms22062914. Int J Mol Sci. 2021. PMID: 33805647 Free PMC article. Review.
References
-
- Glazebrook J. (2005) Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu. Rev. Phytopathol. 43, 205–227 - PubMed
-
- Li L., Zhao Y., McCaig B. C., Wingerd B. A., Wang J., Whalon M. E., Pichersky E., Howe G. A. (2004) The tomato homolog of CORONATINE-INSENSITIVE1 is required for the maternal control of seed maturation, jasmonate-signaled defense responses, and glandular trichome development. Plant Cell 16, 126–143 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

