Regular-spiking cells in the presubiculum are hyperexcitable in a rat model of temporal lobe epilepsy
- PMID: 25210155
- PMCID: PMC11774487
- DOI: 10.1152/jn.00406.2014
Regular-spiking cells in the presubiculum are hyperexcitable in a rat model of temporal lobe epilepsy
Abstract
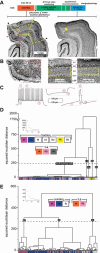
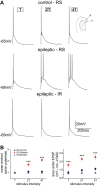
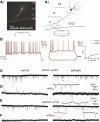
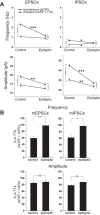
Temporal lobe epilepsy (TLE) is the most common form of adult epilepsy, characterized by recurrent seizures originating in the temporal lobes. Here, we examine TLE-related changes in the presubiculum (PrS), a less-studied parahippocampal structure that both receives inputs from and projects to regions affected by TLE. We assessed the state of PrS neurons in TLE electrophysiologically to determine which of the previously identified cell types were rendered hyperexcitable in epileptic rats and whether their intrinsic and/or synaptic properties were altered. Cell types were characterized based on action potential discharge profiles followed by unsupervised hierarchical clustering. PrS neurons in epileptic animals could be divided into three major groups comprising of regular-spiking (RS), irregular-spiking (IR), and fast-adapting (FA) cells. RS cells, the predominant cell type encountered in PrS, were the only cells that were hyperexcitable in TLE. These neurons were previously identified as sending long-range axonal projections to neighboring structures including medial entorhinal area (MEA), and alterations in intrinsic properties increased their propensity for sustained firing of action potentials. Frequency and amplitude of both spontaneous excitatory and inhibitory synaptic events were reduced. Further analysis of nonaction potential-dependent miniature currents (in tetrodotoxin) indicated that reduction in excitatory drive to these neurons was mediated by decreased activity of excitatory neurons that synapse with RS cells concomitant with reduced activity of inhibitory neurons. Alterations in physiological properties of PrS neurons and their ensuing hyperexcitability could entrain parahippocampal structures downstream of PrS, including the MEA, contributing to temporal lobe epileptogenesis.
Keywords: TLE; cell classification; electrophysiology; hyperexcitability; presubiculum.
Copyright © 2014 the American Physiological Society.
Figures


References
-
- Abbasi S, Kumar SS. Electrophysiological and morphological characterization of cells in superficial layers of rat presubiculum. J Comp Neurol 521: 3116–3132, 2013. - PubMed
-
- Bartesaghi R, Di Maio V, Gessi T. Topographic activation of the medial entorhinal cortex by presubicular commissural projections. J Comp Neurol 487: 283–299, 2005. - PubMed
-
- Bear J, Fountain NB, Lothman EW. Responses of the superficial entorhinal cortex in vitro in slices from naive and chronically epileptic rats. J Neurophysiol 76: 2928–2940, 1996. - PubMed
-
- Boccara CN, Sargolini F, Thoresen VH, Solstad T, Witter MP, Moser EI, Moser MB. Grid cells in pre- and parasubiculum. Nat Neurosci 13: 987–994, 2010. - PubMed
-
- Brenner R, Chen QH, Vilaythong A, Toney GM, Noebels JL, Aldrich RW. BK channel beta4 subunit reduces dentate gyrus excitability and protects against temporal lobe seizures. Nat Neurosci 8: 1752–1759, 2005. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

